Interleukin-18 (Interferon-γ–inducing Factor) Is Produced by Osteoblasts and Acts Via Granulocyte/Macrophage Colony-stimulating Factor and Not Via Interferon-γ to Inhibit Osteoclast Formation (original) (raw)
Abstract
We have established by differential display polymerase chain reaction of mRNA that interleukin (IL)-18 is expressed by osteoblastic stromal cells. The stromal cell populations used for comparison differed in their ability to promote osteoclast-like multinucleated cell (OCL) formation. mRNA for IL-18 was found to be expressed in greater abundance in lines that were unable to support OCL formation than in supportive cells. Recombinant IL-18 was found to inhibit OCL formation in cocultures of osteoblasts and hemopoietic cells of spleen or bone marrow origin. IL-18 inhibited OCL formation in the presence of osteoclastogenic agents including 1α,25-dihydroxyvitamin D3, prostaglandin E2, parathyroid hormone, IL-1, and IL-11. The inhibitory effect of IL-18 was limited to the early phase of the cocultures, which coincides with proliferation of hemopoietic precursors. IL-18 has been reported to induce interferon-γ (IFN-γ) and granulocyte/macrophage colony-stimulating factor (GM–CSF) production in T cells, and both agents also inhibit OCL formation in vitro. Neutralizing antibodies to GM–CSF were able to rescue IL-18 inhibition of OCL formation, whereas neutralizing antibodies to IFN-γ did not. In cocultures with osteoblasts and spleen cells from IFN-γ receptor type II–deficient mice, IL-18 was found to inhibit OCL formation, indicating that IL-18 acted independently of IFN-γ production: IFN-γ had no effect in these cocultures. Additionally, in cocultures in which spleen cells were derived from receptor-deficient mice and osteoblasts were from wild-type mice and vice versa, we identified that the target cells for IFN-γ inhibition of OCL formation were the hemopoietic cells. The work provides evidence that IL-18 is expressed by osteoblasts and inhibits OCL formation via GM–CSF production and not via IFN-γ production.
In the process of osteoclast formation, there is an absolute requirement for cell to cell contact between osteoclastic precursor cells of hemopoietic origin and bone marrow stromal or osteoblastic cells to commit the hemopoietic cell towards osteoclast development (1–3). The osteoclast is a large multinucleated giant cell that contains between 2 and 100 nuclei per cell, expresses tartrate-resistant acid phosphatase (TRAP)1 activity and calcitonin receptors, has the ability to form resorption pits on bone or dentine slices and differs from macrophage polykaryons (4). We developed a coculture system of mouse hematopoietic and primary osteoblastic stromal cells with which to investigate osteoclast development in vitro. In this coculture system, osteoclastlike cells (OCL) are produced in response to a number of systemic or local factors, including 1α,25-dihydroxyvitamin D3 (1α,25(OH)2 D3), prostaglandin E2 (PGE2), parathyroid hormone (PTH), or the interleukins (IL-1, IL-6, and IL-11) (1, 5–7). Generation of these OCLs requires that the osteoblastic and hemopoietic cells are cultured on the same surface (8). These cells are multinucleated and express the OCL characteristics of TRAP activity and calcitonin receptors, and have the capacity to resorb bone (8). In short, they display the properties of mature bona fide osteoclasts. However, the production of such OCLs in cocultures can be inhibited by a number of interleukins (e.g., IL-4, IL-10, and IL-13) and IFN-γ and GM-CSF (9–17).
We previously reported that bone marrow–derived stromal cell lines, MC3T3-G2/PA6 and ST2, had the capacity to support OCL formation in cocultures with hemopoietic cells (18). Recently, we established several bone marrow– derived stromal cell lines from a transgenic mouse and immortalized with a temperature-sensitive variant of the SV40 large T antigen; these cell lines differ in their OCLinductive ability (19, 20). To identify osteoblastic genes that are involved in the process of osteoclastogenesis, we have used differential display PCR (ddPCR) (21) to compare the mRNA populations between OCL-inductive and noninductive cell lines. Using this approach, we identified a recently discovered cytokine, IL-18 (IFN-γ–inducing factor) (22, 23), as a product of osteoblastic stromal cells. Using recombinant IL-18 we showed that it inhibits OCL formation, and we investigated its mode of action.
Materials and Methods
Animals, Cell Lines, and Drugs.
Newborn (0–1-d-old) C57BL/6J mice and 6–9-wk-old male C57BL/6J mice were purchased from Monash University Animal Services Centre (Clayton, Australia). We thank Professor M. Auget (Swiss Institute for Experimental Cancer Research, Switzerland) and Dr. P. Tipping (Monash Medical Centre, Australia) for access to the IFN-γ type II receptor knockout mice (IFN-γ R−/−) (24). The murine stromal cell lines, tsJ2, tsJ10, and tsJ14, were generated by transfection with a retroviral vector expressing a temperature-sensitive variant of the immortalizing gene of SV40 (tsA58; 19, 20). 1α,25(OH)2 D3 was purchased from Wako Pure Chemical Co. (Osaka, Japan). PGE2 was obtained from Sigma Chem. Co. (St. Louis, MO). Recombinant mouse IL-18 and rabbit polyclonal antibodies to mouse IL-18 were prepared as described (22). Recombinant mouse IFN-γ was a gift from Dr. J.A. Hamilton (Department of Medicine, Royal Melbourne Hospital, Australia). Recombinant mouse IL-1β, mouse GM–CSF, and anti-mouse GM–CSF polyclonal antibody were purchased from R&D Systems (Minneapolis, MN). Recombinant human IL-11 was obtained from Dr. T. Willson (Walter and Eliza Hall Institute, Australia). Other chemicals and reagents were of analytical grade.
Coculture System.
Osteoblastic cells were prepared from the calvaria of newborn mice by digestion with 0.1% collagenase (Worthington Biochemical Co., Freefold, Australia) and 0.2% dispase (Godo Shusei, Tokyo, Japan). Bone marrow and spleen cells were obtained from adult and from newborn mice, respectively (6). Osteoblastic cells were cocultured with bone marrow or spleen cells as described (6, 25, 26). In short, primary osteoblastic cells (2 × 104 per well) and nucleated spleen cells (1 × 106 per well) or marrow cells (5 × 105 per well) were cocultured in 48-well plates (Corning Glass Inc., Corning, NY) with 0.4 ml of α-MEM (GIBCO BRL, Gaithersburg, MD) containing 10% fetal bovine serum (Cytosystems, Castle Hill, New South Wales, Australia) in the presence of test chemicals. Cultures were incubated in quadruplicate and cells were replenished on day 3 with fresh medium. OCL formation was evaluated after culturing for 6 to 7 d. Adherent cells were fixed and stained for TRAP, and the number of TRAP-positive OCLs was scored as described (6). For TRAP staining, adherent cells were fixed with 4% formaldehyde in PBS for 3 min. After treatment with ethanol/acetone (50:50 vol/vol) for 1 min, the well surface was air dried and incubated for 10 min at room temperature in an acetate buffer (0.1 M sodium acetate, pH 5.0) containing 0.01% naphthol AS-MX phosphate (Sigma) as a substrate and 0.03% red violet LB salt (Sigma) as a stain for the reaction product in the presence of 50 mM sodium tartrate. TRAPpositive cells appeared dark red. The expression of calcitonin receptors was also assessed by autoradiography using [125I]salmon calcitonin as described (25).
Differential Display PCR.
Total cellular RNA was extracted from cell lines or mouse tissues using guanidine thiocyanate–phenol chloroform and used for reverse transcriptase PCR (RTPCR) essentially as described (27, 28). Differential display PCR (ddPCR) was performed essentially as described (21, 28), except 1 μg of total RNA was reverse transcribed. PCR products were cloned into pCRScript II (Stratagene, La Jolla, CA) or pGEM-T (Promega, Madison, WI). DNA sequence analysis was performed using a T7 sequencingTM kit (Pharmacia Biotech, Uppsala, Sweden). Oligonucleotides were synthesized on an Oligo 1,000M DNA Synthesizer (Beckman Instruments, Inc., Fullerton, CA). The oligonucleotides were the following: for ddPCR, DDMR-2 (5′-CTTGATTGCC-3′) and T12VA (5′-TTTTTTTTTTTT [A,C,G]A-3′); for IL-18 amplification, IL-18-1 (sense strand oligonucleotide 5′-ACTGTACAACCGCAGTAATACGG-3′, nucleotides 286–308 Fig. 1 B) and IL-18-2 (antisense strand oligonucleotide 5′-GGGTATTCTGTTATGGAAATACAGG-3′, nucleotides 804–828; Fig. 1 B), and IL-18-3 (sense strand oligonucleotide 5′-TTGCCAAAAGGAAGATGATG-3′, nucleotides 641–660; Fig. 1 B) served as internal oligonucleotide probe for hybridization studies as described (27, 28); mouse glyceraldehyde3-phosphate dehydrogenase (GAPDH) primers were GAPDH-1, GAPDH-2 (5′-ATGAGGTCCACCACCCTGTT-3′, nucleotides 640–659; 29), and GAPDH-4 as described (30).
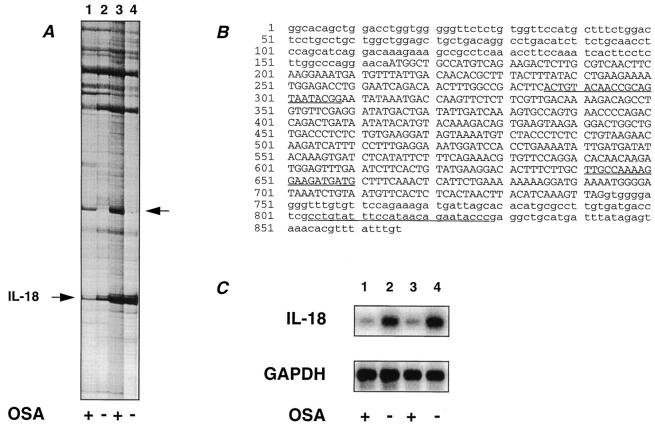
Figure 1.
Identification of IL-18. (A) An example of a ddPCR gel. Lanes correspond to RNA from the different sources: (lane 1) hydrocortisone-treated tsJ10 cells, (lane 2) hydrocortisone-treated tsJ14 cells, (lane 3) 1α,25(OH)2 D3- and PGE2treated tsJ2 cells, and (lane 4) 1α,25(OH)2 D3- and PGE2treated tsJ14 cells. The PCR fragment identified as IL-18 is indicated by the arrow on the left. Indicated by the arrow on the right is a PCR fragment corresponding to a hitherto uncharacterized mRNA species, which is expressed in greater abundance in the OCL-supportive cell lines. The osteoclast-supporting activity (OSA) of these cell lines is indicated below the gel: plus (supportive) or minus (nonsupportive). (B) Nucleotide sequence of mouse IL-18 (GenBankTM accession number D49949). The region corresponding to the differentially expressed PCR fragment isolated from (A) is between nucleotides 636–830. Sequences underlined correspond to oligonucleotides specific to IL-18 used for RT-PCR analysis and detection of RT-PCR products (IL-18-1, IL-18-3, and IL-18-2 from 5′ to 3′). Nucleotides in capitals corrrespond to the coding region of IL-18, whereas those in lower case correspond to the 5′ and 3′ untranslated sequences. (C) Semiquantitative RT-PCR analysis of IL-18 mRNA. PCR products for RNA isolated from different sources was reversed transcribed with oligo (dT) and PCR performed with the primers IL-18-1 and IL-18-2 for 23 cycles, which was in the log-linear range of amplification. Lanes correspond to RNA from (1) hydrocortisone-treated tsJ10 cells, (2) hydrocortisone-treated tsJ14 cells, (3) 1α,25(OH)2 D3- and PGE2-treated tsJ2 cells, and (4) 1α,25(OH)2 D3- and PGE2-treated tsJ14 cells. Resultant PCR products were electrophoresed, transferred to a nylon membrane, and hybridized with a γ-32P–labeled internal detection oligonuleotide for IL-18 (IL-18-3). Similar amplifications for GAPDH with GAPDH-2 and GAPDH-4 for 20 cycles were performed and products detected with γ-32P–labeled GAPDH-1 as previously described (30). The osteoclast-supporting activity (OSA) of these cell lines is indicated below the gel: plus (supportive) or minus (nonsupportive).
Results
Identification of IL-18 mRNA.
To identify stromal cell genes potentially involved in osteoclastogenesis, we have used differential display of eukaryotic mRNA (ddPCR) (21) to identify genes differentially expressed between osteoclastogenic supportive and nonsupportive stromal cell lines. The stromal cell lines were established after immortalization with large T antigen (19, 20) and were stable in their phenotype. The cell lines tsJ2 and tsJ10 could support OCL formation after hydrocortisone (10−6 M) or PGE2 (10−7 M) and 1α,25(OH)2 D3 (10−8 M) treatment. However, in the absence of hydrocortisone or PGE2 and 1α,25(OH)2 D3, the tsJ2 and tsJ10 cell lines were unable to support OCL formation. In contrast, the tsJ14 cell line was unable to support osteoclast formation even in the presence of hydrocortisone or PGE2 and 1α,25(OH)2 D3.
RNA was extracted from OCL-inductive lines (tsJ10 treated with hydrocortisone and tsJ2 treated with PGE2 and 1α,25(OH)2 D3) and from the OCL-noninductive cells (tsJ14 treated with hydrocortisone or with PGE2 and 1α,25(OH)2 D3) and subjected to ddPCR analysis. Using an array of anchored 3′ primers (T12VA, T12VC, T12VG, or T12VT; where V = A, C, and G) and defined 10-mer primers (which act as 5′ primers), several mRNA species appeared to be expressed in a manner consistent with either an OCL-inductive or inhibitory phenotype (see Fig. 1 A). Using the anchored 3′ primer (T12VA) and the 5′ primer DDMR-2, a mRNA species was found to be upregulated in OCL-inductive cells (tsJ10 cells treated with hydrocortisone and tsJ2 cells treated with PGE2 and 1α,25 OH)2 D3; Fig. 1 A, lanes 1 and 3; arrowed at right) with respect to the OCL-noninductive cells (tsJ14 cells treated with hydrocortisone or PGE2 and 1α,25(OH)2 D3; Fig. 1 A, lanes 2 and 4); this mRNA was unique, sharing no identity with sequences in the GenBankTM database, and is currently being analyzed. This primer pair also amplified a mRNA species, which was upregulated in OCL-nonsupportive cells (tsJ14 treated with hydrocortisone; Fig. 1 A, lane 2) compared with OCL-supportive cells (tsJ10 cells treated with hydrocortisone; Fig. 1 A, lane 1). This band of 195 bp was extracted, cloned, and sequenced and was identified as mouse IFNγ–inducing factor (IL-18) (22, 23). The RT-PCR–generated fragment was derived from the 3′ end of the mRNA for IL-18, indicating that the anchored PCR primer had primed near the poly(A) tail (Fig. 1 B). Additionally, the degenerate 10-mer 5′ primer DDMR-2 only differed by one nucleotide from that of the IL-18 cDNA sequence (Fig. 1 B, at nucleotide 640, A versus C, respectively). The band designated by IL-18 in Fig. 1 A appeared to be enhanced by PGE2 and 1α,25(OH)2 D3 as indicated by the amount of amplified product resulting from PCR of RNA from cells treated with these agents (Fig. 1 A, lanes 3 and 4); however, this was complicated by coamplification of another mRNA species under these treatment conditions. Thus, the designated band corresponding to amplified IL-18 cDNA in Fig. 1 A was composed of at least two distinct mRNA species.
To confirm differential expression of IL-18 mRNA in cell lines that were OCL-inductive rather than noninductive, semiquantitative RT-PCR using IL-18–specific oligonucleotides (IL-18-1 and IL-18-2) was undertaken and the PCR product was confirmed by an internal specific oligonucleotide for IL-18 (IL-18-3) (Fig. 1 C). IL-18 mRNA levels were found to be higher in the OCL-noninductive cells (Fig. 1 C, lanes 2 and 4) than the inductive cells (Fig. 1 C, lanes 1 and 3). Quantitation by phosphorimager analysis revealed that IL-18 levels were three- and sevenfold higher in the OCL-noninductive cells (Fig. 1 C, lanes 2 and 4, respectively) than the OCL-inductive cells (Fig. 1 C, lanes 1 and 3). Additionally, semiquantitive RT-PCR established that IL-18 mRNA was expressed in a variety of tissues including brain, heart, liver, lung, spleen, and skeletal muscle (data not shown).
IL-18 Inhibits Osteoclast Formation In Vitro.
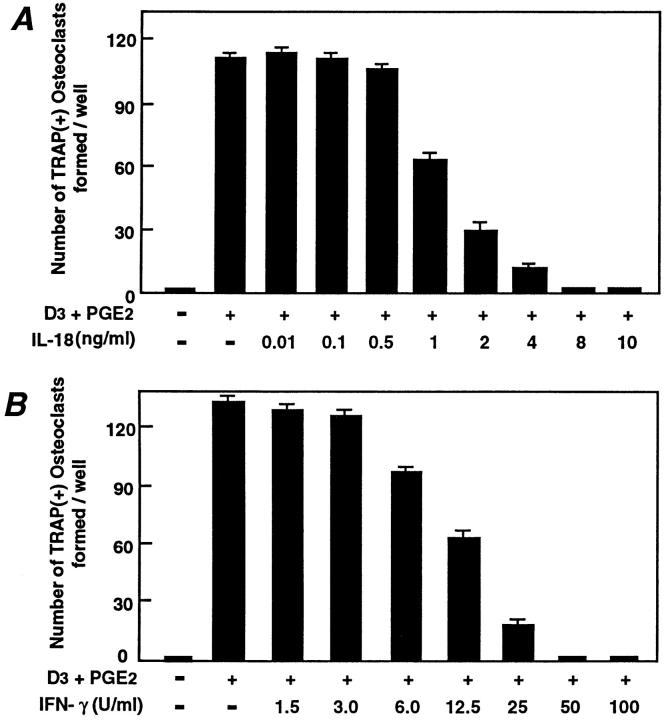
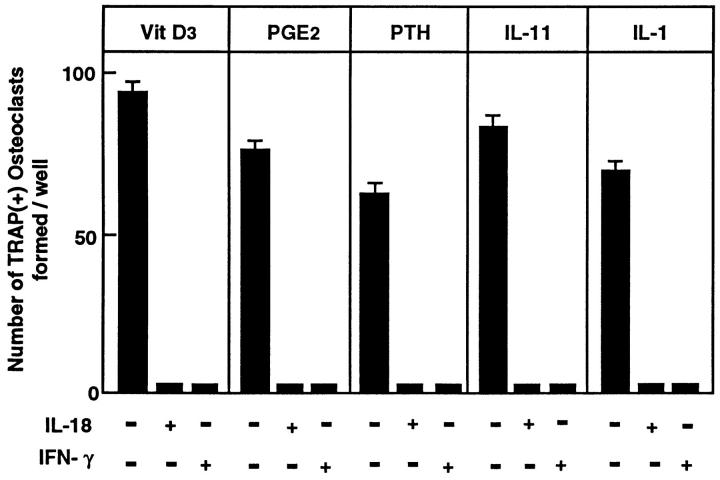
IL-18 was originally identified because of its effect on IFN-γ production, and hence it was originally called interferon-γ–inducing factor (IGIF) (22). The name was subsequently changed to IL-18 (23). Because IFN-γ is a potent inhibitor of osteoclastogenesis (10, 13, 14, 31), and IL-18 mRNA levels were elevated in cells with an OCL-noninductive phenotype, we sought to identify whether IL-18 affects osteoclastogenesis via IFN-γ production. We examined TRAP-positive OCL formation in cocultures of mouse bone marrow and osteoblastic cells. OCLs were formed in cocultures where PGE2 and 1α,25(OH)2 D3 were added; without at least one of these agents, no OCLs were formed (Fig. 2 A). Autoradiographic study using labeled calcitonin revealed that TRAP-positive multinucleated and mononuclear cells formed in these cocultures possessed calcitonin receptors (data not shown). Recombinant mouse IL-18 dose-dependently inhibited OCL formation, giving maximal inhibition at 8 ng/ml (Fig. 2 A); this dose response to IL-18 is very similar to that in other biological systems (22). Recombinant mouse IFN-γ dosedependently inhibited OCL formation with maximal inhibition at 50 U/ml (Fig. 2 B), confirming previous observations in mouse bone marrow cultures (10). In subsequent experiments, IL-18 was used at 10 ng/ml and IFN-γ at 50 U/ml. OCL formation can be enhanced by a number of agents that act through different second messenger systems (e.g., vitamin D receptor, cAMP, and gp130) (1). To examine whether the inhibitory actions of IL-18 on OCL formation was stimulator specific or whether it acted as a general inhibitor, IL-18 was added to cocultures with the osteoclastogenic agents 1α,25(OH)2 D3, PGE2, PTH, or IL-11. IL-18 or IFN-γ inhibited OCL formation induced by each of these agents of osteoclastogenesis (Fig. 3).
Figure 2.
OCL formation in cocultures of mouse bone marrow and osteoblastic cells in the presence of IL-18 (A) or IFN-γ (B). Mouse bone marrow and primary osteoblastic cells were cocultured with 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M) in the presence of increasing concentrations of IL-18 (A) or IFN-γ (B). For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 and PGE2, respectively. After culture for 7 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures, and are representative of three similar experiments.
Figure 3.
Effect of IL-18 (10 ng/ml) on OCL formation in cocultures of mouse bone marrow and osteoblastic cells in the presence of 1α,25(OH)2 D3 (10−8 M), PGE2 (10−7 M), PTH (200 ng/ml), IL-11 (20 ng/ml), and IL-1 (100 ng/ml). After culture for 7 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures and are representative of three similar experiments.
IL-18 Inhibits the Early Stage of OCL Formation.
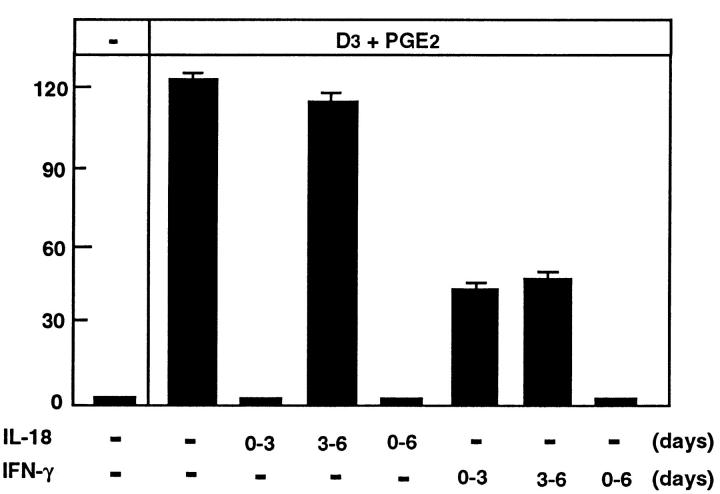
Next, we addressed the action of IL-18 and IFN-γ on the process of OCL formation. Treatment of cocultures revealed that the inhibitory actions of IL-18 on OCL formation occurred during the first 3 d of coculture (proliferating period), whereas IL-18 had no effect during the last 3 d of coculture (Fig. 4). In contrast, although IFN-γ inhibited OCL formation over the entire coculture period (days 0–6), it inhibited OCL formation by 50% during both the proliferating period (days 0–3) and the differentiation phase (days 3–6) (Fig. 4). These results suggested a fundamental difference between the inhibitory effects of IL-18 and IFN-γ, although potential stimulation of IFN-γ production by IL-18 could not be excluded by these experiments alone.
Figure 4.
Effect of IL-18 and IFN-γ on OCL formation in cocultures of mouse bone marrow and osteoblastic cells during the coculture period in the absence and presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M). IL-18 (10 ng/ml) and IFN-γ (50 U/ml) were present over the entire culture period (days 0–6) or during the first 3 d (0–3) or the last 3 d (3–6). Media change occurred at day 3 of the culture. After culture for 6 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. This experiment was repeated on two further occasions with similar results.
IL-18 Acts by Inhibitory Mechanism Distinct from IFN-γ.
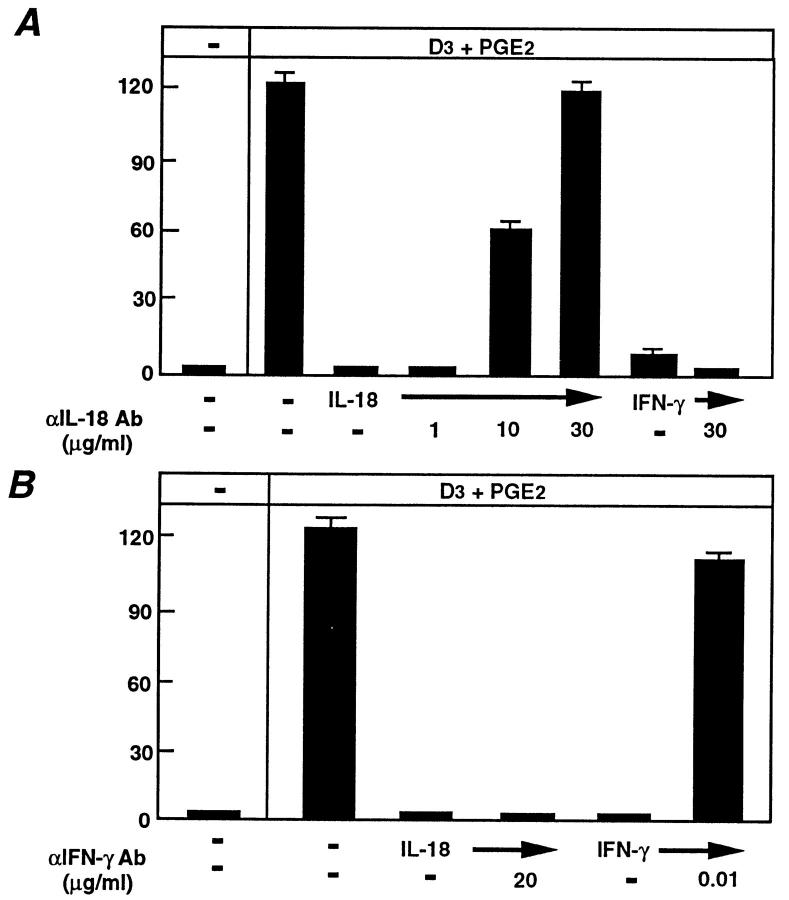
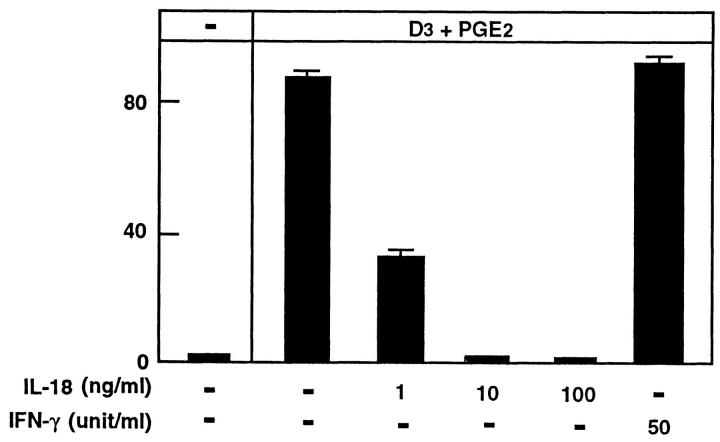
Next, we sought to distinguish the inhibitory mechanisms of IL-18 and IFN-γ by using neutralizing antibodies to either IL-18 or IFN-γ in coculture experiments. We initially titrated the two antibodies (α-IL-18 antibody or α-IFN-γ antibody) and determined the concentration at which each antibody could rescue either IL-18–induced inhibition of OCL formation (for α-IL-18 antibody) or IFN-γ–induced inhibition of OCL formation (for α-IFN-γ antibody). A polyclonal neutralizing IL-18 antibody (at 30 μg/ml) rescued IL-18 inhibition of OCL formation (Fig. 5 A), however, this antibody (at 30 μg/ml) did not rescue the IFN-γ inhibition of OCL formation (Fig. 5 A). Because IFN-γ was speculated to be a downstream effector molecule of IL-18, we did not expect the neutralizing IL-18 antibody to rescue IFN-γ inhibition of OCL formation. The monoclonal IFN-γ neutralizing antibody at very low concentration (1 ng/ml) rescued IFN-γ inhibitory effects, whereas even at 2,000-fold excess of the IFN-γ rescuing concentration (20 μg/ml) it did not protect against IL-18 inhibition of OCL formation (Fig. 5 B). The inability of the IFN-γ–neutralizing antibody to rescue OCL formation after IL-18 treatment further suggested that IL-18 was not acting through IFN-γ. Finally, we made use of IFN-γ R−/−) mice (24). Cocultures of osteoblastic cells and spleen cells derived from the IFN-γ R−/− mouse were treated with either IL-18 or IFN-γ. IFN-γ, as expected, was unable to elicit an inhibition of OCL formation in these cocultures due to the absence of its cognate receptor on any cells (Fig. 6). In contrast, IL-18 inhibited OCL formation in these cocultures in a dose-dependent manner with a maximal effect at 10 ng/ml (Fig. 6), which is similar to normal mice cocultures as described above (see Fig. 2 A). This served as definitive proof that IL-18, although capable of inducing IFN-γ (a known inhibitor of OCL formation), mediated its inhibitory action on OCLs via an IFN-γ–independent mechanism.
Figure 5.
Effect of neutralizing antibodies against IL-18 or IFN-γ in rescuing OCL formation in cocultures of mouse bone marrow and osteoblastic cells treated with IL-18 or IFN-γ. Cocultures were incubated in the presence or absence of IL-18 (10 ng/ml) and IFN-γ (50 U/ml) and the effect of antibodies against IL-18 (A) or IFN-γ (B) were determined. For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M), respectively. After culture for 7 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. This experiment was repeated twice.
Figure 6.
OCL formation in cocultures of spleen cells and osteoblastic cells derived from IFN-γ receptor type II knockout (IFN-γ R −/−) mice. IL-18 (10 ng/ml) or IFN-γ (50 U/ml) were present over the entire culture period. For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M), respectively. After culture for 7 d, TRAP-positive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. This experiment was repeated twice.
IFN-γ Effects on OCL Formation in Cocultures from IFN-γ R− /− Mouse.
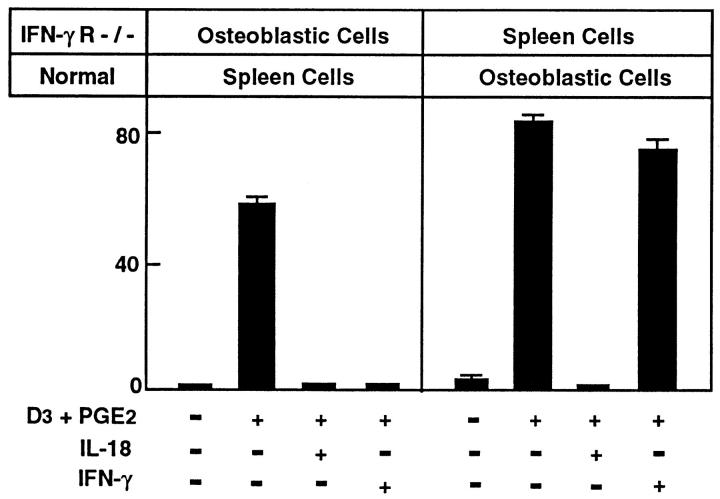
The IFN-γ R−/− mouse also provided a unique opportunity to address which cells in the cocultures were sensitive to the actions of IFN-γ. We performed cocultures using osteoblastic cells from the IFN-γ R−/−mouse with spleen cells from a normal C57BL/6J mouse and the reciprocal experiment, that is, osteoblastic cells from a normal mouse with spleen cells from the IFN-γ R−/− mouse, and treated these cocultures with IL-18 or IFN-γ (Fig. 7). As expected, IL-18 inhibited OCL formation under both culture regimes. However, IFN-γ inhibited OCL formation when cocultures were performed with normal spleen cells and osteoblasts from IFN-γ R−/− mice, but did not inhibit OCL formation in cocultures using normal osteoblasts with spleen cells from IFN-γ R−/− mice. These results indicated that IFN-γ acts specifically on osteoclastic precursors in spleen cells to inhibit osteoclast formation and not via the osteoblastic/stromal cells.
Figure 7.
OCL formation in cocultures of normal C57/BL6 mousederived spleen cells with osteoblastic cells derived from IFN-γ R−/− mice and cocultures of normal C57BL/J6 mouse-derived osteoblastic cells with spleen cells derived from IFN-γ R−/− mice. Cocultures were performed in the presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M) and treated with IL-18 (10 ng/ml) or IFN-γ (50 U/ml). For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 and PGE2, respectively. After culture for 7 d, TRAPpositive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. This experiment was repeated twice.
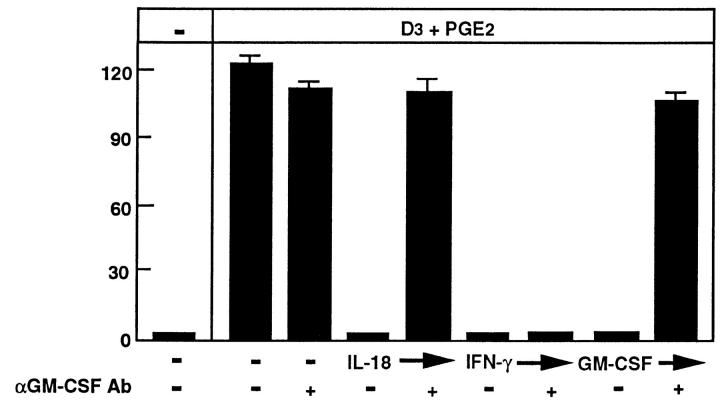
IL-18 Inhibits OCL Formation Via GM–CSF.
In addition to enhancing IFN-γ production, IL-18 has been shown to induce GM–CSF production in mitogen-stimulated peripheral blood mononuclear cells (23). Like IFN-γ, GM– CSF is a potent inhibitor of osteoclast formation in the mouse system (16, 26, 32–34), suggesting that GM–CSF may also be a candidate molecule involved in the IL-18 inhibition of OCL formation. Recombinant mouse GM– CSF (0.1 ng/ml) was found to inhibit OCL formation in this coculture system (Fig. 8). Thus, we examined whether a neutralizing antibody to GM–CSF could rescue the inhibitory effect of IL-18 on OCL formation. The polyclonal antibody (at 10 μg/ml) alone had no effect on OCL formation, but did indeed rescue OCL formation from the inhibitory actions of GM–CSF and those of IL-18 (Fig. 8). Moreover, the concentration of this antibody (0.1–1.0 μg/ml) to rescue OCL formation as a result of GM–CSF or IL-18 inhibition was similar. The neutralizing antibody to GM– CSF (10 μg/ml) was unable to rescue the IFN-γ inhibition of OCL formation (Fig. 8). This result, combined with the previous experiments, indicates that IL-18 most likely inhibits OCL formation by increasing the production of GM–CSF, a known inhibitor of OCL formation, and not via IFN-γ.
Figure 8.
Effect of neutralizing antibodies against GM–CSF to rescue OCL formation in cocultures of mouse bone marrow and osteoblastic cells treated with GM–CSF (0.1 ng/ml), IL-18 (10 ng/ml), or IFN-γ (50 U/ml). Cocultures were incubated in the presence or absence of GM– CSF, IL-18, or IFN-γ and the effect of neutralizing antibodies to GM– CSF (1 μg/ml) were determined. For negative and positive controls, cocultures were performed in the absence and presence of 1α,25(OH)2 D3 (10−8 M) and PGE2 (10−7 M), respectively. After culture for 7 d, TRAPpositive OCLs were counted. Data are expressed as the means ± SEM of quadruplicate cultures. Similar results were obtained with three repeat experiments.
Discussion
In this report, we establish that a recently discovered cytokine, IL-18, can inhibit OCL formation in mouse cocultures. Elevated expression of mRNA for IL-18 was observed in stromal cell lines that were unable to support OCL formation, and recombinant IL-18 was a potent inhibitor of OCL formation in vitro. The discovery of IL-18 resulted from its ability to induce IFN-γ production by T cells (22). Although IFN-γ is known to inhibit osteoclast formation in vitro (4), the present experiments exclude IFN-γ as an intermediate in the inhibitory effect of IL-18 on osteoclast formation, for a number of reasons.
First, antibodies against IFN-γ failed to rescue IL-18 inhibition of OCL formation in cocultures, although they were able to prevent the effect of IFN-γ itself. Second, in our experiments with cells from type II IFN-γ R−/− mice, IL-18 still manifested its inhibitory action on osteoclast formation. Furthermore, an effect of IL-18 had no effect on the production of macrophages in vitro (Udagawa, N., and J.A. Hamilton, unpublished data), nor did IL-18 influence bone resorption by freshly isolated osteoclasts (Holloway, W., and M.T. Gillespie, unpublished data). These latter two sets of observations contrast with the effects of IFN-γ, which promotes the proliferation of macrophages in vitro (35) and inhibits resorption by freshly isolated osteoclasts (36). Finally, our finding that IFN-γ submaximally inhibited OCL formation during both the proliferative (days 0–3) and the maturation (days 3–6) phases of the coculture indicated that the inhibitory actions of IL-18 on OCL formation do not involve IFN-γ as an intermediate.
On the other hand, these results indicate that GM–CSF might be a molecular intermediate in the inhibitory effect of IL-18 on osteoclastogenesis. This arises from the fact that a neutralizing antibody against GM–CSF was able to prevent IL-18 inhibition of OCL formation. Furthermore, the finding that the effect of IL-18 in our experiments was on the early phase of the cocultures, in which proliferation of hemopoietic precursors is the dominant process, is entirely consistent with GM–CSF involvement. Conflicting reports exist for the action of GM–CSF on the production of osteoclasts in vitro. In human bone marrow cultures, GM– CSF stimulated the formation of multinucleated cells (37), whereas in mouse marrow cultures GM–CSF inhibited OCL formation (16, 26, 32–34). Our results in the cocultures are in accordance with the latter.
IL-18 shares substantial identity with IL-1, including the IL-1 signature-like sequence (F-X(12)-F-X-S-X(6)-F-L; 23) and secondary structure (38). These properties suggested that IL-18 may be functionally similar to the IL-1 family of cytokines. Thus, it was foreseeable that IL-18 may bind to the IL-1 receptor and could potentially inhibit OCL formation in a manner akin to the IL-1 receptor antagonist (39). Indeed, the suggestion was made that IGIF (22) should be called IL-1γ (39). However, attempts to displace IL-18 inhibition of OCL formation with excess of IL-1β were unsuccessful, indicating that either IL-18 binds to a receptor distinct from that for IL-1 or that it has a higher affinity for the IL-1 receptor than IL-1β. The identity of the IL-18 receptor remains to be established.
The finding that IL-18 mRNA was elevated in OCLnoninductive stromal cells, and that IL-18 could inhibit OCL formation, implies that IL-18, and by inference GM– CSF, participates in local control of osteoclastogenesis. Thus, the regulation of IL-18 and GM–CSF expression in the bone microenvironment by circulating hormones or by other local factors may function to limit the generation of osteoclasts.
Acknowledgments
This work was supported by a Program Grant from the National Health and Medical Research Council (NHMRC) Australia (T.J. Martin and M.T. Gillespie) and by the Medical Research Council (T.J. Chambers).
Footnotes
M.T. Gillespie is a Research Fellow of the NHMRC Australia and N. Udagawa was a recipient of a C.R. Roper Fellowship from The University of Melbourne.
N. Udagawa and N.J. Horwood contributed equally to this work.
1 Abbreviations used in this paper: 1α,25(OH)2 D3, 1α,25-dihydroxyvitamin D3; αGM–CSF, neutralizing antibody to GM–CSF; αIFN-γ, neutralizing antibody to IFN-γ; ddPCR, differential display PCR; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GM–CSF, granulocyte/macrophage colony-stimulating factor; IFN-γ R−/−, IFN-γ receptor type II knockout; IGIF, interferon-γ-inducing factor; OCL, osteoclast-like multinucleated cells; PGE2, prostaglandin E2; PTH, parathyroid hormone; RT, reverse transcription (or transcribed); TRAP, tartrate resistant acid phosphatase.
References
- 1.Suda, T., N. Takahashi, and T.J. Martin. 1995. Modulation of osteoclast differentiation: update. In Endocrine Review Monographs. Vol. 4. D.D. Bikle and A. Negrovilar, editors. Endocrine Society, Bethesda, MD. 266–270.
- 2.Martin TJ, Ng KW. Mechanisms by which cells of the osteoblast lineage control osteoclast formation and activity. J Cell Biochem. 1994;56:357–366. doi: 10.1002/jcb.240560312. [DOI] [PubMed] [Google Scholar]
- 3.Suda, T., N. Udagawa, and N. Takahashi. 1996. Cells of bone: osteoclast generation. In Principles of Bone Biology. J.P. Billezikian, L.G. Raisz, G.A. Rodan, editors. Academic Press, San Diego, CA. 87–102.
- 4.Roodman GD. Advances in bone biology: the osteoclast. Endocrine Rev. 1996;17:308–332. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- 5.Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, Koishihara Y, Ohsugi Y, Kumaki K, Taga T, et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci USA. 1993;90:11924–11928. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udagawa N, Takahashi N, Katagiri T, Tamura T, Wada S, Findlay DM, Martin TJ, Hirota H, Taga T, Kishimoto T, et al. IL-6 induction of osteoclast differentiation depends upon IL-6 receptors expressed on osteoblastic cells, but not on osteoclast progenitors. J Exp Med. 1995;182:1461–1468. doi: 10.1084/jem.182.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romas E, Udagawa N, Zhou H, Tamura T, Saito M, Taga T, Hilton DJ, Suda T, Ng KW, Martin TJ. The role of gp130-mediated signals in osteoclast development: regulation of interleukin 11 production by osteoblasts and distribution of its receptor in bone marrow cultures. J Exp Med. 1996;183:2581–2592. doi: 10.1084/jem.183.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocrine Rev. 1992;13:66–88. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- 9.Shioi A, Teitelbaum SL, Ross FP, Suzuki H, Ohara J, Lacey DL. Interleukin 4 inhibits murine osteoclast formation in vitro. J Cell Biochem. 1991;47:272–277. doi: 10.1002/jcb.240470313. [DOI] [PubMed] [Google Scholar]
- 10.Lacey DL, Erdmann JM, Teitelbaum SL, Tan H-L, Ohara J, Shioi A. Interleukin 4, interferon-γ, and prostaglandin E impact the osteoclastic cell-forming potential of murine bone marrow macrophages. Endocrinology. 1995;136:2367–2376. doi: 10.1210/endo.136.6.7750457. [DOI] [PubMed] [Google Scholar]
- 11.Xu LX, Kukita T, Kukita A, Otsuka T, Niho Y, Iijima T. Interleukin-10 selectively inhibits osteoclastogenesis by inhibiting differentiation of osteoclast progenitors into preoteoclast-like cells. J Cell Physiol. 1995;165:624–629. doi: 10.1002/jcp.1041650321. [DOI] [PubMed] [Google Scholar]
- 12.McHugh, K.P., S.L. Teitelbaum, and F.P. Ross. 1995. Interleukin-13, like interleukin-4, modulates murine osteoclastogenesis and expression of the integrin α,β3 on osteoclast precursors. J. Bone Miner. Res. 10(Suppl.):S487.
- 13.Takahashi N, Mundy GR, Roodman GD. Recombinant human interferon-γ inhibits formation of human osteoclast-like cells. J Immunol. 1986;137:3544–3549. [PubMed] [Google Scholar]
- 14.Horowitz, M.C., and J.A. Lorenzo. 1996. Local regulators of bone: IL-1, TNF, lymphotoxin, interferon-γ, IL-8, IL-10, IL-4, the LIF/IL-6 family, and additional cytokines. In Principles of Bone Biology. J.P. Billezikian, L.G. Raisz, G.A. Rodan, editors. Academic Press, San Diego, CA. 687–700.
- 15.Nakano Y, Watanabe K, Morimoto I, Okada Y, Ura K, Sato K, Kasono K, Nakamura T, Eto S. Interleukin-4 inhibits spontaneous and parathyroid hormone– related protein-stimulated osteoclast formation in mice. J Bone Miner Res. 1994;9:1533–1539. doi: 10.1002/jbmr.5650091005. [DOI] [PubMed] [Google Scholar]
- 16.Hattersley G, Chambers TJ. The effects of interleukin 3, granulocyte–macrophage– and macrophage–colony stimulating factors on osteoclast differentiation from mouse hemopoietic tissue. J Cell Physiol. 1990;142:201–209. doi: 10.1002/jcp.1041420125. [DOI] [PubMed] [Google Scholar]
- 17.Owens JM, Gallagher AC, Chambers TJ. IL-10 modulates formation of osteoclasts in murine hemopoietic cultures. J Immunol. 1996;157:936–940. [PubMed] [Google Scholar]
- 18.Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T. The bone marrow–derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- 19.Chambers TJ, Owens JM, Hattersley G, Jat PS, Noble MD. Generation of osteoclast-inductive and osteoclastogenic cell lines from the H-2KbtsA58transgenic mouse. Proc Natl Acad Sci USA. 1993;90:5578–5582. doi: 10.1073/pnas.90.12.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens JM, Gallagher AC, Chambers TJ. Bone cells required for osteoclastic resorption but not for osteoclastic differentiation. Biochem Biophys Res Commun. 1996;222:225–229. doi: 10.1006/bbrc.1996.0726. [DOI] [PubMed] [Google Scholar]
- 21.Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science (Wash DC) 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 22.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature (Lond) 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 23.Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, et al. Cloning of the cDNA for human IFN-γ-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. [PubMed] [Google Scholar]
- 24.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science (Wash DC) 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi N, Udagawa N, Akatsu T, Tanaka H, Shionome M, Suda T. Role of colony-stimulating factors in osteoclast development. J Bone Miner Res. 1991;6:977–985. doi: 10.1002/jbmr.5650060912. [DOI] [PubMed] [Google Scholar]
- 27.Southby J, Murphy LM, Martin TJ, Gillespie MT. Cell-specific and regulator-induced promoter usage and messenger ribonucleic acid splicing for parathyroid hormone–related protein. Endocrinology. 1996;137:1349–1357. doi: 10.1210/endo.137.4.8625910. [DOI] [PubMed] [Google Scholar]
- 28.Traianedes K, Findlay DM, Martin TJ, Gillespie MT. Modulation of the signal recognition particle 54-kDa subunit (SRP54) in rat preosteoblasts by the extracellular matrix. J Biol Chem. 1995;270:20891–20894. doi: 10.1074/jbc.270.36.20891. [DOI] [PubMed] [Google Scholar]
- 29.Tso JY, Sun X-H, Kao T-h, Reece KS, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexicity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suda N, Gillespie MT, Traianedes K, Zhou H, Ho PWM, Hards DK, Allan EH, Martin TJ, Moseley JM. Expression of parathyroid hormone–related protein in cells of osteoblast lineage. J Cell Physiol. 1996;166:94–104. doi: 10.1002/(SICI)1097-4652(199601)166:1<94::AID-JCP11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 31.Kurihara N, Roodman GD. Interferon-α and -γ inhibit interleukin-1 β-stimulated osteoclast-like cell formation in long-term human marrow cultures. J Interferon Res. 1990;10:541–547. doi: 10.1089/jir.1990.10.541. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi N, Udagawa N, Akatsu T, Tanaka H, Isogai Y, Suda T. Deficiency of osteoclasts in osteopetrotic mice is due to a defect in the local microenvironment provided by osteoblastic cells. Endocrinology. 1991;128:1792–1796. doi: 10.1210/endo-128-4-1792. [DOI] [PubMed] [Google Scholar]
- 33.Shinar DM, Sato M, Rodan GA. The effect of hemopoietic growth factors on the generation of osteoclastlike cells in mouse bone marrow cultures. Endocrinology. 1990;126:1728–1735. doi: 10.1210/endo-126-3-1728. [DOI] [PubMed] [Google Scholar]
- 34.Shuto T, Kukita T, Hirata M, Jimi E, Koga T. Dexamethasone stimulates osteoclast-like cell formation by inhibiting granulocyte–macrophage colony-stimulating factor production in mouse bone marrow cultures. Endocrinology. 1994;134:1121–1126. doi: 10.1210/endo.134.3.8119150. [DOI] [PubMed] [Google Scholar]
- 35.Vairo G, Argyriou S, Knight KR, Hamilton JA. Inhibition of colony-stimulated macrophage proliferation by tumor necrosis factor-α, IFN-γ, and lipopolysaccharide is not due to a general loss of responsiveness to growth factor. J Immunol. 1991;146:3469–3477. [PubMed] [Google Scholar]
- 36.Gowen M, Nedwin GE, Mundy GR. Preferential inhibition of cytokine-stimulated bone resorption by recombinant interferon gamma. J Bone Miner Res. 1986;1:469–474. doi: 10.1002/jbmr.5650010511. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald BR, Mundy GR, Clark S, Wang EA, Kuehl TJ, Stanley ER, Roodman GD. Effects of human recombinant CSF–GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J Bone Mineral Res. 1986;1:227–233. doi: 10.1002/jbmr.5650010210. [DOI] [PubMed] [Google Scholar]
- 38.Bazan JF, Timans JC, Kastelein RA. A newly defined interleukin-1? . Nature (Lond) 1996;379:591. doi: 10.1038/379591a0. [DOI] [PubMed] [Google Scholar]
- 39.Kitazawa R, Kimble RB, Vannice JL, Kung VT, Pacifici R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest. 1994;94:2397–2406. doi: 10.1172/JCI117606. [DOI] [PMC free article] [PubMed] [Google Scholar]