Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide (original) (raw)
. Author manuscript; available in PMC: 2013 Dec 10.
Published in final edited form as: Nature. 1997 Dec 18;390(6661):10.1038/37803. doi: 10.1038/37803
Abstract
Cyclic-nucleotide-gated (CNG) channels in outer segments of vertebrate photoreceptors generate electrical signals in response to changes in cyclic GMP concentration during phototransduction1. CNG channels also allow the influx of Ca2+, which is essential for photoreceptor adaptation2. In cone photoreceptors, cGMP triggers an increase in membrane capacitance indicative of exocytosis, suggesting that CNG channels are also involved in synaptic function3. Here we examine whether CNG channels reside in cone terminals and whether they regulate neurotransmitter release, specifically in response to nitric oxide (NO), a retrograde transmitter that increases cGMP synthesis and potentiates synaptic transmission in the brain4–6. Using intact retina, we show that endogenous NO modulates synapses between cones and horizontal cells. In experiments on isolated cones, we show directly that CNG channels occur in clusters and are indirectly activated by _S_-nitrosocysteine (SNC), an NO donor. Furthermore, both SNC and pCPT–cGMP, a membrane-permeant analogue of cGMP, trigger the release of transmitter from the cone terminals. The NO-induced transmitter release is suppressed by guanylate cyclase inhibitors and prevented by direct activation of CNG channels, indicating that their activation is required for NO to elicit release. These results expand our view of CNG channel function to include the regulation of synaptic transmission and mediation of the presynaptic effects of NO.
Patch-clamp experiments were performed on acutely dissociated cones from lizard retina, used because they possess large (5–10 μm diameter) presynaptic terminals (labelled T in Fig. 1a). By varying the duration of proteolytic enzyme treatment and trituration, we observed either intact cones or cones devoid of outer segments and/or presynaptic terminals. To investigate whether CNG channels are present in the terminals we applied the whole-cell patch-clamp configuration to cones containing terminals and cones lacking terminals. All the cones selected for use in these experiments were devoid of outer segments, to eliminate their contribution to the whole-cell CNG current. To ensure that the cone terminals were intact and functional, we applied depolarizing voltage pulses to assay for the presence of a voltage-gated Ca2+ current, which has been previously characterized in cones with healthy terminals3,7,8.
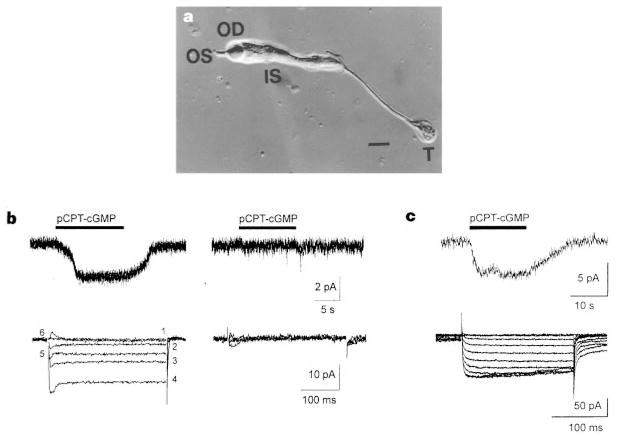
Figure 1.
Responses of cone terminals to pCPT–cGMP. a, Acutely dissociated cone photoreceptor from Anolis carolinensis retina. Note the outer segment (OS), oil droplet (OD), inner segment (IS), and presynaptic terminal, or ‘pedicle’, of the cone (T). Scale bar, 10 μm. b, Records from a cone containing a terminal (left) and from a cone lacking a terminal (right). Only the cone with the terminal exhibits an inward current in response to 100 μM pCPT–cGMP (top traces; holding potential = −60 mV). Similarly, only the cone with the terminal exhibits voltage-gated Ca2+ currents in response to 20 mV incremental depolarizations from −60 mV to +40 mV (bottom traces). c, An isolated terminal exhibits current in response to 100 μM pCPT–cGMP and 10 mV incremental depolarizations from −60 to +30 mV. Tail currents result from Ca2+-activated Cl− channels7,31, owing to the presence of 140 mM Cl− in the pipette, rather than 30 mM Cl− plus 110 mM aspartate, as in b.
The results of these experiments are shown in Fig. 1b. All cones exhibiting a voltage-gated Ca2+ current also exhibited an inward current when the membrane-permeant cGMP analogue pCPT–cGMP (8-para-chlorothio-cGMP) was applied (9 of 9 cells). In contrast, none of the cones without terminals exhibited either the voltage-gated Ca2+ current or pCPT–cGMP-activated current (0 of 8 cells). To confirm that the pCPT–cGMP-activated current is specifically localized to the terminal, ‘whole-terminal’ recordings were obtained after isolating the terminal from the inner segment by transecting the axon with a glass probe. Isolated terminals exhibited both the voltage-gated Ca2+ current and the pCPT–cGMP-activated current (Fig. 1c).
Steady-state current–voltage (I–V) relations show that the conductance activated by pCPT–cGMP is distinct from the voltage-gated Ca2+ conductance (Fig. 2). Application of pCPT–cGMP selectively activates a voltage-independent conductance with a reversal potential near 0 mV, without affecting the voltage-gated Ca2+ conductance. In contrast, nifedipine reduces but does not completely block the voltage-gated Ca2+ conductance, as reported previously8, but has no effect on the pCPT–cGMP-activated conductance.
Figure 2.
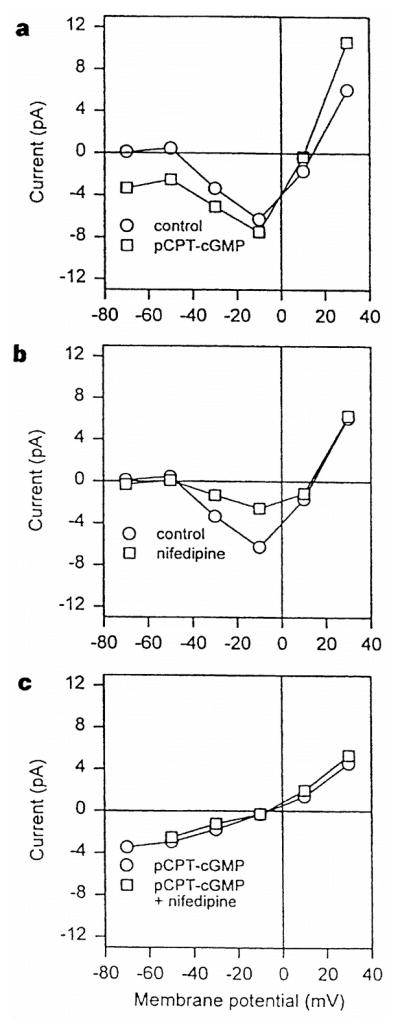
Steady-state I–V curves of the pCPT–cGMP-activated and voltage-gated Ca2+ currents. a, I–V curve in normal saline (control) and after application of 100 μM pCPT–cGMP. b, I–V curve in normal saline and after application of 1 μM nifedipine. c, I–V relation of the pCPT–cGMP-activated conductance obtained by subtraction of the I–V curve in control saline from the I–V curve in pCPT–cGMP, both obtained in the absence (circles) or presence (squares) of 1 μM nifedipine. All currents were measured at the end of 200-ms voltage pulses from a holding potential of −60 mV.
To determine whether the conductance activated by pCPT–cGMP results directly from activation of CNG channels or indirectly from other effects of cGMP, such as activation of cGMP-dependent protein kinase, we applied cGMP onto excised inside-out patches from cone terminals in the absence of ATP, GTP or added kinases. A recording from a patch containing several CNG channels directly activated by cGMP is shown in Fig. 3a. Like other CNG channels1, these channels exhibited voltage-dependent block after the addition of divalent cations (1 mM Ca2+ or Mg2+; data not shown), which accounts for the fact that the currents observed in patches under divalent-free conditions were much larger than the whole-cell currents obtained with divalents present. Dose–response curves fitted with the Hill equation (Fig. 3b) showed that the CNG channels in excised patches had an unusually low sensitivity to cGMP with a _K_1/2 of activation of 206 ± 23 μM (n = 9), but up to 2 mM cAMP caused no activation of the CNG channels. CNG channels in patches excised from outer segments of these cones had a similar sensitivity, with a _K_1/2 for cGMP of 159 ± 29 μM (n = 6). Both the terminal and outer-segment CNG channels were much more sensitive to pCPT–cGMP with a _K_1/2 of activation of 10.3 ± 3.9 μM (n = 5), consistent with the higher sensitivity of other CNG channels for this analogue9.
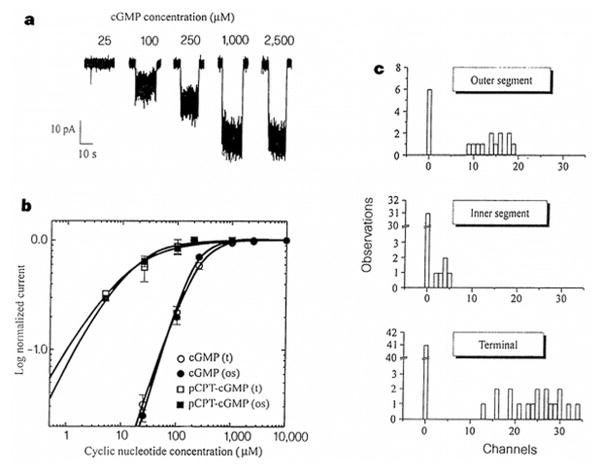
Figure 3.
CNG channels in cone terminals. a, Direct activation of CNG channels in an inside-out patch excised from a cone terminal. The patch contained an estimated 29 channels. b, Dose–response curves for cone terminal (t) and outer segment (os) CNG-channel activation by cGMP and pCPT–cGMP. Continuous curves show fits to the Hill equation. c, Distribution of CNG channels within cones.
To investigate further the subcellular distribution of CNG channels, excised patches were made from outer segments, inner segments, and terminals of cones (Fig. 3c). Most patches (67%) from outer segments exhibited CNG channels. Moreover, these patches always contained many channels (9–19 per patch). In contrast, patches from inner segments rarely exhibited CNG channels (14%), and those that did always contained a low density of channels (2–5 channels per patch). Hence, as has been shown in rods10, CNG channels in cones are much more densely packed in outer segments than in inner segments. Patches from the terminals, like those from inner segments, rarely contained CNG channels (31%), but those that did contained a high density of channels (13–34 channels per patch). Taken together with the whole-cell data, these patch results suggest that CNG channels are clustered in cone terminals. CNG channels would be ideally positioned to influence neurotransmitter release if these clusters were located near release sites.
What regulates the activity of CNG channels in cone terminals? CNG channels in terminals might be constitutively activated by resting levels of cGMP, like CNG channels in rod and cone outer segments, which are kept active in the dark by cytoplasmic levels of cGMP1. In addition, neurotransmitters released onto cone terminals from other retinal cells might alter the activity of CNG channels. Nitric oxide (NO) is a strong candidate to be a retinal transmitter that could modulate the activity of cone terminal CNG channels, for several reasons. First, NO synthase is found in rod and cone photoreceptors, and also in processes of bipolar and glial cells in the outer plexiform layer of the retina, adjacent to terminals of photoreceptors11,12. Second, in rods, NO has been shown to modulate the voltage-gated Ca2+ conductance and a voltage-independent conductance12. Finally, the soluble form of guanylate cyclase, which synthesizes cGMP, is activated by NO and is found predominantly in inner, rather than outer, segments of cones13.
To test whether NO affects synaptic transmission from cone photoreceptors, we exposed intact retinas from tiger salamanders to flashes of light, and examined the voltage response of horizontal cells, which receive input from cones (Fig. 4a, b). The hyperpolarizing light response of horizontal cells exhibits a delayed depolarizing sag that is partly dependent on feedback inhibition of cone terminals by the horizontal cells themselves14–16. Incubation of retina with a competitive inhibitor of NOS, NG-nitro-L-arginine (L-NNA), resulted in a dose-dependent suppression of the sag in the horizontal cell response, as compared with control. Incubation of retina with L-arginine, the normal substrate of NOS, had no effect on the sag response, but prevented the inhibition caused by L-NNA. Incubation with ODQ, a specific inhibitor of nitric oxide-sensitive guanylate cyclase17, also significantly reduced the sag, although to a lesser extent. These results indicate that endogenous production of NO in the retina modulates synaptic communication between cones and horizontal cells, in part by stimulating the synthesis of cGMP.
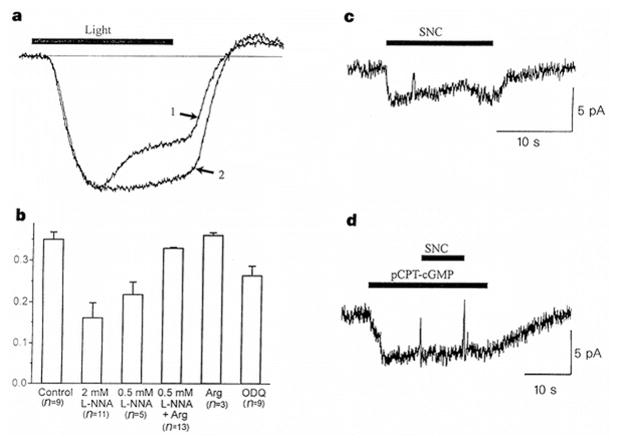
Figure 4.
Modulation of cone synapses by NO. a, Endogenous NO modulates synaptic communication between cones and horizontal cells. Voltage responses from horizontal cells incubated in normal saline (trace 1) or in 2 mM L-NNA (trace 2) to 500-ms steps of full-field illumination (2 × 10−7 μW μm−2). Control and L-NNA responses were normalized to peak amplitudes (15 and 10 mV, respectively). b, Summary of feedback response experiments, showing mean ± s.e.m. of the ‘response sag’, defined as 1 − (S/P), where P is the peak voltage and S is the voltage at the end of the light step. Recordings were made from horizontal cells in retinae super-fused for 1 h with control saline, L-NNA, 2 mM L-arginine, 2 mM L-arginine + L-NNA, or 100 μM ODQ, as indicated. Results with ODQ (P < 0.02) or L-NNA alone (P < 0.001) were statistically different from control. c, Activation of CNG current in an isolated lizard cone terminal by 50 μM SNC. d, Occlusion of the SNC response by prior application of 100 μM pCPT–cGMP.
Does NO alter neurotransmitter release from cone terminals by activating CNG channels? To determine whether NO causes the activation of CNG channels, we applied S-nitrosocysteine (SNC), an NO donor, onto isolated lizard cone terminals. We found that SNC activates an inward current (Fig. 4c) associated with a voltage-insensitive conductance with a reversal potential near 0 mV (data not shown). Moreover, the SNC-elicited current is mimicked and occluded by pCPT–cGMP (Fig. 4d), suggesting that SNC and pCPT–cGMP converge to activate the same population of ion channels. Similar results were observed in a total of four experiments. Application of NO-depleted SNC solution (prepared >12 h before use) had no effect on the conductance of cones (n = 4).
As well as indirectly activating CNG channels by stimulating cGMP synthesis, NO directly activates CNG channels in olfactory neurons by covalently modifying the channel protein18. In our experiments, SNC applied directly onto excised inside-out patches from cone terminals failed to activate CNG channels and had no effect on the sensitivity of the channels to cGMP (n = 5). Therefore, it is likely that NO acts by stimulating cGMP production to activate the cone terminal CNG channels.
Does the regulation of CNG channels by NO lead to an increase in neurotransmitter release from cones? To monitor release of the cone neurotransmitter glutamate19, we used cultured horizontal cells from catfish retina as biosensors20 (Fig. 5a). A dissociated cone devoid of its outer segment was manipulated so that its terminal directly contacted the horizontal cell, which was monitored with a whole-cell patch pipette. The horizontal cells generate a detectable response to as little as 0.5 μM glutamate through the activation of NMDA and non-NMDA receptors, and little desensitization is observed during prolonged (5 s) applications of glutamate (Fig. 5b). Owing to slow changes in the holding current, responses to low glutamate concentrations (<2 μM) more reliably induced increases in the current variance (‘noise’) than changes in the mean current. Application of pCPT–cGMP or SNC onto the cone terminal–horizontal cell pair elicited a reversible increase in current variance (Fig. 5c, d). This increase in variance was blocked by application of 1 μM kynurenic acid, which blocks glutamate responses in horizontal cells20. Moreover, although isolated horizontal cells sometimes responded to pCPT–cGMP and SNC with an outward shift in the holding current, there was no increase in noise unless the cone terminal was present. Hence the increase in the current variance results from release of glutamate from the cone terminal.
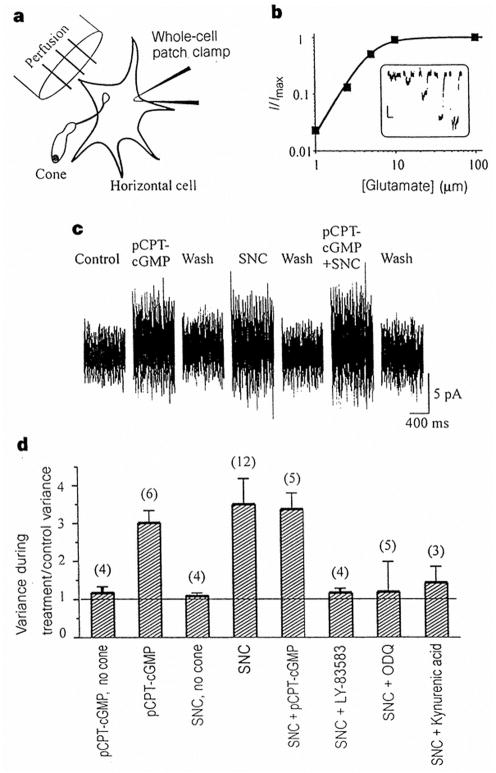
Figure 5.
Biosensor measurements of transmitter release from cone terminals. a, Experimental arrangement. b, Sensitivity of the glutamate response of the horizontal cell biosensor. Bath application of 1, 2.5, 5, 10 and 100 μM glutamate elicits the responses shown in the inset (vertical scale 10 pA, horizontal scale 10 s). The graph shows the Hill fit to peak responses with a _K_1/2 of 4.9 μM. c, Steady-state changes in current noise in a horizontal cell contacted by the terminal of a dissociated cone. Recordings show reversible effects of 100 μM pCPT–cGMP and 50 μM SNC applied alone, and pCPT–cGMP and SNC applied together. d, Summary of release experiments. Ratio of horizontal-cell current variance during treatment to control variance. Bars show mean variance ±s.e.m., with number of cells tested in parentheses. Results for pCPT–cGMP, SNC and SNC + pCPT–cGMP were statistically different from control (P < 0.001).
The results of these release experiments are summarized in Fig. 5d. Both pCPT-cGMP and SNC elicited three- to fourfold increases in current variance. Coapplication of pCPT–cGMP and SNC resulted in no further increase in variance, suggesting that NO triggers release through the same mechanisms as pCPT–cGMP. However, application of 10 μM ODQ or 10 μM LY-83583, both inhibitors of soluble guanylate cyclase17,21, blocked the action of SNC. Hence the increase in transmitter release elicited by SNC seems to be dependent upon cGMP synthesis. Because cGMP selectively activates CNG channels, these results suggest that the increase in glutamate release elicited by NO is mediated by CNG channels.
Cone terminals process two types of signals: those generated in the outer segment in response to light and those elicited by feedback transmitters released from adjacent retinal cells. Although the signal relayed from the outer segment to the terminal is crucial for rapid light responses, it is the feedback onto cone terminals that modulates the gain of the cone outputs synapse and mediates colour opponency and surround responses, and thereby underlies an important early step in the processing of visual information16. The long diffusion distance between the outer segment and the terminal makes it unlikely that CNG channels in the terminal are affected by the decrease in cGMP resulting from phototransduction. However, the activation threshold of voltage-gated Ca2+ channels in cones (above −45 mV; Fig. 2 and refs 3, 7, 22) may not fully account for the voltage sensitivity of synaptic transmission from cones during light responses, which is apparent even at −70 mV (refs 3, 23). Our results show that CNG channels are present in high-density clusters in cone terminals. These channels may be tonically activated by steady-state levels of cGMP and contribute to transmitter release by allowing Ca2+ influx even at hyperpolarized potentials. These CNG channels also mediate the effects of NO on neurotransmitter release from cones. NO5,24 and cGMP25 also increase neurotransmitter release in the hippocampus, where they have been implicated in mediating long-term potentiation of synaptic transmission. The recent discovery of CNG channels in the hippocampus26,27 and elsewhere in the central nervous system28 raises the possibility that the mechanism we have described here may be more widely used in the brain as a retrograde signalling pathway to modulate synaptic transmission.
Methods
Electrophysiological recording from cones
Retinas from lizards (Anolis sagrei, collected locally, or A. carolinensis, from Nasco, Fort Atkinson, WI) were isolated, treated with papain, and triturated to obtain dissociated cones, as described7. Cells were placed in saline containing (in mM): 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 5 glucose and 10 NaHEPES, pH 7.5. For whole-cell recordings, patch pipettes contained (in mM): 110 Cs aspartate, 30 CsCl, 0.5 MgCl2, 5 EGTA, 0.1 GTP, 0.1 ATP and 10 CsHEPES, pH 7.5, which isolated CNG and voltage-gated Ca2+ current from K+ and Cl− currents. Patch-clamp recordings were made with an Axopatch 200A (Axon Instruments) and data were analysed using p-Clamp software. All experiments were performed at 21–23 °C. For excised patch experiments, both the patch pipette and the perfusion solution contained (in mM): 155 NaCl, 5 KCl, 5 EGTA and 5 NaHEPES, pH 7.5. Perfusion solutions were administered at 1–2 ml min−1. Cyclic GMP (Sigma) and 8-_p_-chlorophenylthio-guanosine 3′,5′-monophosphate (pCPT–cGMP; BioLog) were prepared daily from concentrated stock solutions.
Analysis of channel density and distribution
To estimate the minimum number of active CNG channels in excised patches, the total cGMP-activated conductance was determined at saturating cGMP (2 mM) and divided by the single-channel conductance (21 pS) determined in separate experiments. Single-channel analysis showed that the maximal open probability of the cone CNG channels was high (>90%), indicating that the ratio of total conductance to single-channel conductance not only indicates the minimum number of channels, but is a good estimate of the absolute channel number29. In the analysis of channel distribution in cones, open excised patches were distinguished from resealed membrane vesicles by examining current noise and patch resistance in the absence of cGMP. In 205 patches from all regions of the cone there was a bimodal distribution of these parameters, with patches in one group exhibiting root mean squared noise levels <0.25 pA and resistances >2.5 GΩ. Application of cGMP never elicited channel activity in these patches. We assume that these represent recordings from resealed vesicles; they have been disregarded from further analysis.
Biosensor measurements of glutamate release
Cultures of cone-driven horizontal cells from the retina of catfish (Ictalurus punctatus) were prepared as described20. After 1–5 days in culture, culture dishes containing the cells were transferred to the electrophysiology set-up and acutely dissociated Anolis cones were added. An isolated cone or a group of 2–3 cones were manipulated to bring their terminals into direct contact with the horizontal cell. Whole-cell patch-clamp recordings were obtained from the horizontal cell and 0.5–100 μM glutamate was applied. Only cells with detectable responses to ≤1 μM glutamate were used. The whole-cell pipette solution contained (in mM): 110 CsCl, 2 MgCl2, 0.5 CaCl2, 5 EGTA, 5 Na2ATP and 10 NaHEPES, pH 7.5. cGMP modulates ionic currents in horizontal cells by the activation of cGMP-dependent protein kinase (PKG)30. KT-5823 (10 μM; Calbiochem), a PKG inhibitor, blocks these effects, so it was also included in the whole-cell patch solution. Horizontal cells were held at −40 mV and currents filtered at 5 kHz. Current records were analysed using Origin (Microcal) for determination of current variance. Statistical differences were evaluated using one-way analysis of variance.
Intact retina experiments
Tiger salamanders (Ambystoma tigrinum) maintained at 5 °C were killed and pithed and the eyes removed under dim illumination. The cornea, lens and iris were removed and the resulting retinal eyecup allowed to dark-adapt for at least 1 h. Glass microelectrodes filled with 3 M K-acetate and 200 mM KCl (150–200 MΩ) were used for intracellular recording from horizontal cells. Voltage responses to light flashes were amplified with a WPI M707 Microprobe system and analysed using Basic-Fastlab software. Solutions at 21–23 °C were continuously superperfused at 1 ml min−1. Control saline contained (in mM): 95 NaCl, 2.5 KCl, 3 CaCl2, 1.5 MgCl2, 30 NaHCO3 and 1 mg ml−1 glucose, and was bubbled continuously with 95% O2/5% CO2 to maintain a pH of 7.6. Each recording was obtained from individual horizontal retinal cells exposed for 1 h to control saline, saline containing NG-nitro-L-arginine (L-NNA; Calbiochem). L-arginine (Sigma) or 1_H_-[1,2,4]oxadiazolo[4,3-_a_]quinoxalin-1-one (ODQ; Tocris).
Acknowledgments
We thank D. Eng for help with preliminary experiments; S. Nawy and R. Bookman for comments; and D. Dixon for advice on horizontal cell culture. This work was supported by grants from the NIH and the American Heart Association (R.H.K.), and the MRC Canada and the AHFMR (S.B.).
References
- 1.Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 2.Koutalos Y, Yau KW. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- 3.Rieke F, Schwartz EA. A cGMP-gated current can control exocytosis at cone synapses. Neuron. 1994;13:863–873. doi: 10.1016/0896-6273(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 4.Garthwaite J, Charles SJ, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 5.O’Dell TJ, Hawkins RD, Kandel ER, Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci USA. 1991;88:11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuman EM, Madison DV. Nitric oxide and synaptic function. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- 7.Maricq AV, Korenbrot JI. Calcium and calcium-dependent chloride currents generate action potentials in solitary cone photoreceptors. Neuron. 1988;1:503–515. doi: 10.1016/0896-6273(88)90181-x. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson MF, Barnes S. The dihydropyridine-sensitive calcium channel subtypes in cone photoreceptors. J Gen Physiol. 1996;107:621–630. doi: 10.1085/jgp.107.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman AL, Yamanaka G, Eckstein F, Baylor DA, Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci USA. 1985;82:8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe SI, Matthews G. Regional distribution of cGMP-activated ion channels in the plasma membrane of the rod photoreceptor. J Neurosci. 1988;8:2334–2337. doi: 10.1523/JNEUROSCI.08-07-02334.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liepe BA, Stone C, Koistinaho J, Copenhagen DR. Nitric oxide synthase in Muller cells and neurons of salamander and fish retina. J Neurosci. 1994;14:7641–7654. doi: 10.1523/JNEUROSCI.14-12-07641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurenny DR, et al. Modulation of ion channels in rod photoreceptors by nitric oxide. Neuron. 1995;13:315–324. doi: 10.1016/0896-6273(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 13.Koch KW, Lambrecht HG, Haberecht M, Redburn D, Schmidt HH. Functional coupling of a Ca2+/calmodulin-dependent nitric oxide synthase and a soluble guanylyl cyclase in vertebrate photoreceptor cells. EMBO J. 1994;13:3312–3320. doi: 10.1002/j.1460-2075.1994.tb06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baylor DA, Fuortes MGF, O’Bryan PM. Receptive fields of single cones in the retina of the turtle. J Physiol (Lond ) 1971;214:265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccolino M, Neyton J, Gerschenfeld HM. Centre-surround antagonism in small-field luminosity horizontal cells of turtle retina. J Neurophysiol. 1981;45:363–375. doi: 10.1152/jn.1981.45.3.363. [DOI] [PubMed] [Google Scholar]
- 16.Wu SM. Synaptic transmission in the outer retina. Annu Rev Physiol. 1994;56:141–168. doi: 10.1146/annurev.ph.56.030194.001041. [DOI] [PubMed] [Google Scholar]
- 17.Garthwaite J, et al. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 18.Broillet MC, Firestein S. Direct activation of the olfactory cyclic nucleotide-gated channel through modification of sulfhydryl groups by NO compounds. Neuron. 1996;16:377–385. doi: 10.1016/s0896-6273(00)80055-0. [DOI] [PubMed] [Google Scholar]
- 19.Copenhagen DR, Jahr CE. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989;341:536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- 20.Tachibana M, Okada T. Release of endogenous excitatory amino acids from ON-type bipolar cells isolated from the goldfish retina. J Neurosci. 1991;11:2199–2208. doi: 10.1523/JNEUROSCI.11-07-02199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulsch A, Busse R, Liebau S, Forstermann U. LY83583 interferes with the release of endothelium-derived relaxing factor and inhibits soluble guanylate cyclase. J Pharmacol Exp Ther. 1988;247:282–288. [PubMed] [Google Scholar]
- 22.Barnes S, Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol. 1989;94:719–743. doi: 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Normann RA, Perlman I. Signal transmission from red cones to horizontal cells in the turtle retina. J Physiol (Lond ) 1979;286:509–524. doi: 10.1113/jphysiol.1979.sp012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meffert MK, Premack BA, Schulman H. Nitric oxide stimulates Ca2+-independent synaptic vesicle release. Neuron. 1994;12:1235–1244. doi: 10.1016/0896-6273(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 25.Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
- 26.Kingston PA, Zufall F, Barnstable CJ. Rat hippocampal neurons express genes for both rod retinal and olfactory cyclic nucleotide-gated channels: novel targets for cAMP/cGMP function. Proc Natl Acad Sci USA. 1996;93:10440–10445. doi: 10.1073/pnas.93.19.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley J, et al. Functional expression of the heteromeric “olfactory” cyclic nucleotide-gated channel in the hippocampus: a potential effector of synaptic plasticity in brain neurons. J Neurosci. 1997;17:1993–2005. doi: 10.1523/JNEUROSCI.17-06-01993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn JT, Grunwald ME, Yau KW. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- 29.Horn R. Estimating the number of channels in patch recordings. Biophys J. 1991;60:433–439. doi: 10.1016/S0006-3495(91)82069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon DB, Copenhagen DR. Metabotropic glutamate receptor-mediated suppression of an inward rectifier current is linked via a cGMP cascade. J Neurosci. 1997 doi: 10.1523/JNEUROSCI.17-23-08945.1997. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes S, Deschenes MC. Contribution of Ca and Ca-activated Cl channels to regenerative depolarization and membrane bistability of cone photoreceptors. J Neurophysiol. 1992;68:745–755. doi: 10.1152/jn.1992.68.3.745. [DOI] [PubMed] [Google Scholar]