Loss of the Plasma Membrane-Bound Protein Gas1p in Saccharomyces cerevisiae Results in the Release of β1,3-Glucan into the Medium and Induces a Compensation Mechanism To Ensure Cell Wall Integrity (original) (raw)
Abstract
Deletion of GAS1/GGP1/CWH52 results in a lower β-glucan content of the cell wall and swollen, more spherical cells (L. Popolo, M. Vai, E. Gatti, S. Porello, P. Bonfante, R. Balestrini, and L. Alberghina, J. Bacteriol. 175:1879–1885, 1993; A. F. J. Ram, S. S. C. Brekelmans, L. J. W. M. Oehlen, and F. M. Klis, FEBS Lett. 358:165–170, 1995). We show here that _gas1_Δ cells release β1,3-glucan into the medium. Western analysis of the medium proteins with β1,3-glucan- and β1,6-glucan-specific antibodies showed further that at least some of the released β1,3-glucan was linked to protein as part of a β1,3-glucan–β1,6-glucan–protein complex. These data indicate that Gas1p might play a role in the retention of β1,3-glucan and/or β-glucosylated proteins. Interestingly, the defective incorporation of β1,3-glucan in the cell wall was accompanied by an increase in chitin and mannan content in the cell wall, an enhanced expression of cell wall protein 1 (Cwp1p), and an increase in β1,3-glucan synthase activity, probably caused by the induced expression of Fks2p. It is proposed that the cell wall weakening caused by the loss of Gas1p induces a set of compensatory reactions to ensure cell integrity.
The cell wall of Saccharomyces cerevisiae is a supramolecular structure that determines the shape of the cell and is responsible for its mechanical strength. It consists of four main components that are synthesized and modified either by plasma membrane-bound complexes (chitin and β1,3-glucan) or by enzymes in the secretory pathway (cell wall mannoproteins and possibly also β1,6-glucan) (for reviews, see references 29 and 39). An important component of the cell wall is the glucose polymer β1,3-glucan. The β1,3-glucan synthase activity is localized at the inner side of the plasma membrane and activated by GTP-bound Rho1p (10, 36, 44). Multiple approaches have led to the identification of a putative membrane-bound subunit of a β1,3-glucan synthase complex. The gene FKS1 (8, 11) was also cloned as CND1 (15), CWH53 (46), ETG1 (7), GSC1 (21), and PBR1 (3), and it encodes a large protein of 215 kDa with multiple transmembrane helices. Loss of the gene resulted in a dramatic reduction in β1,3-glucan synthase activity (8, 21, 41), as well as a reduction in β1,3-glucan content (3, 46). An alternative subunit of the β1,3-glucan synthase complex was cloned by homology to FKS1. The homolog FKS2/GSC2 is 88% identical to FKS1. Disruption of either FKS1 or FKS2 yields viable cells, but simultaneous disruption is lethal (21, 35), indicating that they have overlapping functions. Transcription of FKS1 is cell cycle regulated (35, 46) and predominates during growth on glucose (35). FKS2 is expressed in the absence of glucose and is induced by the addition of Ca2+. Disruption of FKS1 induces the expression of FKS2, which is responsible for the residual glucan synthase activity in an _fks1_Δ strain (35).
β1,3-Glucans are synthesized as linear molecules that are extruded into the periplasmic space. In a mature cell wall, however, most of the β1,3-glucan is branched (34), covalently linked to chitin (30), or covalently linked to β1,6-glucan and β1,6-glucan-containing cell wall mannoproteins (13, 27, 37). The formation of branch points and cross-links presumably takes place outside the plasma membrane by transglucosylation reactions catalyzed by extracellular enzymes.
The formation of a rigid cell wall requires proper cross-linking of the cell components. We therefore anticipated that mutations leading to defects in cross-linking would affect cell wall integrity. To search for such genes, we carried out a genetic screen for mutants hypersensitive to Calcofluor White (45). Calcofluor White is known to interfere with the extracellular assembly of cell wall components. One of the isolated mutants, cwh52, was shown to be identical to GAS1/GGP1 (46). This gene encodes an abundant 125-kDa glycoprotein anchored to the external face of the plasma membrane by a glycosylphosphatidylinositol (GPI) anchor (38, 52). We will refer to this gene as GAS1 for the remainder of this paper. The function of Gas1p is unknown. Deletion of GAS1 was not lethal but resulted in an apparently lower β-glucan content of the cell wall (46) and a more spherical morphology (42). In view of the localization of Gas1p at the extracellular side of the plasma membrane, these data suggest a possible role for Gas1p in the incorporation of β1,3-glucan in the cell wall. Here we show that disruption of GAS1 results in the release of β1,3-glucan into the medium, indicating that Gas1p is indeed involved in the incorporation of β1,3-glucan in the cell wall. Several phenotypes that pointed to a possible secondary strengthening of the cell wall were observed in a _gas1_Δ mutant. Those secondary phenotypes were not specific for the _gas1_Δ mutant but were also found in other mutants affected in the synthesis or assembly of β1,3-glucan, such as fks1 and knr4 mutants. We suggest that these secondary phenotypes are part of a general compensatory mechanism that comes into action when the cell wall is weakened.
MATERIALS AND METHODS
Yeast and bacterial strains and growth conditions.
The strains used in this study were ARC99.4A (MATα cwh52-2 ura3-52), ARC42.7D (MATα cwh53-1 ura3-52) (45), FY833 (MATa his3Δ300 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63), FY834 (MATα his3Δ300 ura3-52 leu2Δ1 lys2Δ202 trp1Δ63) (54), AR100 (MATα fks1::HIS3 in FY834), and AR104 (MATa gas1::LEU2 in FY833). Growth conditions and growth media were as described elsewhere (45). To assay Calcofluor White hypersensitivity, a spot assay was used. Cells were diluted or concentrated to an _A_530 of 7.0 (108 cells/ml). Subsequently, a 10-fold dilution series was made, and 5 μl of each cell suspension, starting with the 10-fold-diluted suspension, was spotted on a series of yeast extract-peptone-dextrose (YPD) plates containing increasing concentrations of Calcofluor White. Plates containing 0, 5, 10, 25, 50, and 100 μg/ml were routinely used. Growth was scored after 2 days at 28°C. Standard procedures were used for genetic crosses, sporulation of diploids, and dissection of tetrads (47). Yeast transformations were made by the lithium acetate method (22). Escherichia coli DH5α was used for propagation of all plasmids.
Plasmids, DNA purification, and recombinant DNA techniques.
Plasmids YDp-L and YDp-H (2) were used to amplify the LEU2 and HIS3 genes by PCR. Plasmid DNA was prepared from E. coli as described elsewhere (48). Yeast DNA was isolated by the method of Hoffman and Winston (18). Specific DNA probes were randomly labeled by using [α-32P]dATP (Amersham) as a substrate (12). DNA probes were purified by using a prepacked G25 Sephadex column (Pharmacia). DNA fragments were isolated from agarose gels with a GeneClean II kit (Bio 101, La Jolla, Calif.).
PCR amplification.
The PCR amplifications (with a Perkin-Elmer Cetus DNA Thermal Cycler) to obtain deletion fragments were performed in a total volume of 100 μl containing 5 U of Super Taq polymerase (HT Biotechnology, Ltd.), 10 μl of 10× Super Taq buffer, 0.2 mM each deoxynucleoside triphosphate, 20 pmol of each primer, and 3 ng of plasmid DNA. The reaction mixture was incubated for 1 min at 95°C and submitted to 4 cycles of PCR (1 min at 94°C, 1 min at 45°C, and 2 min at 72°C), followed by 35 cycles (1 min at 94°C and 2 min at 72°C). In the final step, the extension step lasted 10 min.
Construction of deletion mutants.
Gene deletions were performed by the method of Baudin et al. (1a). DNA fragments containing the HIS3 gene (1.3 kb) or the LEU2 gene (1.9 kb) were prepared by PCR using the corresponding YDp plasmids (2) as template DNA. For each gene deletion, two primers were designed for the amplification of the auxotrophic markers. Each primer was composed of two regions. The 3′ region (17 or 18 nucleotides) was identical to the DNA flanking the marker genes on the YDp plasmids. Since the different marker genes contain identical flanking regions, disruption of the same gene with different markers could be performed with the same set of primers. The remainder of each primer (50 nucleotides) corresponded to a specific region within the target gene. The PCR product, the auxotrophic marker gene flanked by 50 nucleotides of the target gene, was precipitated and directly used for transformation. Correct integrations were confirmed by genomic Southern analysis (51). To generate a GAS1/CWH52 deletion mutant, 1,029 nucleotides of the GAS1 locus (from nucleotide 100 to 1129) were deleted and replaced with a 1.9-kb DNA fragment containing the LEU2 gene. The FKS1/CWH53 deletion mutant was constructed by replacing 3,030 nucleotides of the FKS1/CWH53 locus (from nucleotide 340 to 3370) with the HIS3 gene.
RNA analysis.
Total RNA was isolated from exponentially growing cells (optical density at 530 nm [OD530], 1 to 1.5) in YPD medium by the acidic phenol method as described in Current Protocols in Molecular Biology (CD-ROM) (1). After being run on a 1% agarose gel in 20 mM MOPS [3-(_N_-morpholino)propanesulfonic acid]–5 mM sodium acetate–1 mM EDTA–2.4% formamide, the RNA samples were transferred to Hybond-N+ (Amersham) nylon membranes by capillary blotting with 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at room temperature. The transferred RNA was cross-linked to the membrane by a 4-min exposure to UV light of 254 nm and was hybridized with [α-32P]dATP (Amersham)-labeled probes. A 492-bp DNA fragment containing the CWP1 region and a 604-bp DNA fragment containing the TIP1 region were used to detect CWP1 and TIP1 transcripts, respectively, and were kindly provided by Marcel van der Vaart (Unilever Research Laboratories, Vlaardingen, The Netherlands). Actin transcripts were detected with a 25-mer oligonucleotide labeled by 5′-end phosphate labeling by using fast protein liquid chromatography-pure polynucleotide kinase (Pharmacia) according to the instructions of the manufacturer.
Analysis of cell wall sugar composition.
Isolation of cell walls from early-log-phase cells (OD530, between 1.0 and 2.0) and determination of their sugar composition by high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) were performed as described previously (45). For performance of carbohydrate analysis of whole cells, early-log-phase cells were washed three times with 25 ml of 10 mM Tris-HCl (pH 7.8). The cell pellet was dried in order to determine the dry weight. Cells corresponding to 60 μg (dry weight) were hydrolyzed in 400 μl of 2 M trifluoroacetic acid (TFA) for 4 h at 100°C, and after the evaporation of the TFA, the monosaccharides were separated and quantified by using the HPAEC-PAD system as described previously (45).
Measurement of β1,3-glucan in the growth medium.
Cells were grown in synthetic medium to late exponential phase (OD530 of 4.0, corresponding to about 3 × 107 cells/ml). Cells were removed by centrifugation, and the medium was filtered over a 0.22-μm-pore-size filter. To measure the amount of β1,3-glucan released into the culture medium, a competitive enzyme immunoassay was performed (9). Wells of a microtiter plate were coated with laminarin (β1,3-glucan) (2 μg/ml in phosphate-buffered saline [PBS]). After a wash with PBS, culture medium and β1,3-glucan-specific antibodies were added. β1,3-Glucan antibodies not bound to the microtiter plate were washed away, and bound antibodies were detected with horse anti-rabbit peroxidase-conjugated antibodies. Concentrations of β1,3-glucan in the medium were determined by using known concentrations of laminarin as standards.
Isolation of medium proteins.
Cells were grown in synthetic medium buffered with 50 mM morpholineethanesulfonic acid (MES) to pH 6.0, with the appropriate amino acids and uracil, at 28°C and were harvested in the exponential phase (OD530, between 2 and 3). Cells were separated from the culture medium by centrifugation (for 10 min at 2,000 × g), and the pelleted cells were used for cell wall isolation (45). The growth medium was centrifuged again for 10 min at 2,000 × g. The medium proteins were precipitated by the trichloroacetic acid-sodium desoxycholate precipitation procedure according to Ozols (40). Protein concentrations were determined with the bicinchoninic acid-protein assay reagent (Pierce, Rockford, Ill.) with bovine serum albumin (BSA) as the standard.
Isolation of cell wall proteins.
Isolated cell walls (150 mg [fresh weight]) were extracted with 750 μl of sodium dodecyl sulfate (SDS) extraction buffer to remove noncovalently bound mannoproteins (37). Subsequently, walls were washed five times with water to remove the SDS. To isolate laminarinase-extractable mannoproteins, 30 mg (fresh weight) of walls was washed once with 50 mM sodium acetate (pH 5.5) and resuspended in 60 μl of the same buffer (0.5 mg/μl). Fifteen microliters of mollusc laminarinase (2 mg/ml; Sigma) was added, and after incubation for 2 h at 37°C, another 15 μl of laminarinase was added, followed by an additional 2 h of incubation. To obtain Quantazyme-extractable mannoproteins, 30 mg of SDS-extracted and water-washed cell walls was washed once more with 50 mM Tris-HCl (pH 7.5). Cell walls were resuspended in the same buffer (0.5 mg/μl) and incubated for 4 h with 60 U of Quantazyme ygl (Quantum Biotechnologies, Inc., Montreal, Canada) in 50 mM Tris-HCl (pH 7.5).
Enzymatic treatments of medium proteins.
Medium proteins (7.5 μg) were digested for 2 h at 37°C with Quantazyme (final concentration, 1 U/μl) in 50 mM Tris-HCl (pH 7.5) in a total volume of 20 μl. Similarly, 7.5 μg of proteins was digested for 2 h at 37°C with either laminarinase (final concentration, 0.5 μg/μl) or pure endo-β-1,6-glucanase II (final concentration, 0.4 mU/μl) isolated from Trichoderma harzianum (4) in 50 mM sodium acetate (pH 5.5) in a total volume of 20 μl. Purified endo-β-1,6-glucanase II was kindly provided by Jesus de la Cruz and Antonio Llobell (Institute for Plant Biochemistry and Photosynthesis, University of Seville, Seville, Spain).
Analysis of cell wall and medium proteins.
Cell wall and medium proteins were separated by linear-gradient (2.2 to 20%) polyacrylamide gel electrophoresis (PAGE) (32). Gels were either silver stained for proteins (5) or blotted by electrophoretic transfer onto Immobilon polyvinylidene difluoride membranes (Millipore, Etten-Leur, The Netherlands) for Western analysis. The anti-β1,6-glucan antiserum was raised against BSA–β1,6-glucan conjugates in rabbits (37) and was purified by affinity chromatography (33). Similarly, the anti-β1,3-glucan antiserum was raised against BSA–β1,3-glucan conjugates and affinity purified (9, 26). The membranes were blocked with 5% (wt/vol) milk powder in PBS and were incubated with anti-β1,6-glucan antiserum or anti-β1,3-glucan antiserum. Both glucan antisera were used in a dilution of 1:5,000 in PBS containing 3% (vol/vol) BSA (26, 37). Membranes were also incubated with a polyclonal antiserum raised against Cwp1p (50), in a serum dilution of 1:2,500 in PBS containing 3% (vol/vol) BSA. For immunodetection with the β1,3-glucan antiserum and the Cwp1p antiserum, membranes were treated for 30 min with 50 mM periodic acid–100 mM sodium acetate (pH 4.5) prior to the blocking in order to enhance the binding of the antiserum (49). The binding of the antisera was determined with goat anti-rabbit immunoglobulin G–peroxidase by using enhanced chemiluminescence detection reagents (Amersham). Preparation of the Fks1p- and Fks2p-specific antibodies, and conditions for Western analysis, were as described by Mazur et al. (35).
In vitro β1,3-glucan synthase activity.
β1,3-Glucan synthase activities were assayed in cells grown on glucose as described previously (8). The specific activity was determined from the rate of accumulation of labeled glucose from UDP-glucose into trichloroacetic acid-precipitable material over the time interval from 60 to 120 min and is given as nanomoles per hour per milligram of protein.
RESULTS
Gas1p is involved in the retention of β1,3-glucan.
We have previously shown that mutations in the GAS1/CWH52 and FKS1/CWH53 genes resulted in a Calcofluor White-hypersensitive phenotype and a relative reduction of β-glucan compared to chitin and mannan (46). As observed with the original gas1/cwh52-1 and fks1/cwh53-1 mutants, disruption of the genes also resulted in a Calcofluor White-hypersensitive phenotype (data not shown) and a relative reduction in β-glucan (see below). Whereas FKS1 encodes a putative subunit of the β1,3-glucan synthase complex (8, 21), a possible role for Gas1p is still unknown. Given the localization of Gas1p at the extracellular side of the plasma membrane (38), we examined a role for Gas1p in the incorporation of β1,3-glucan into the cell wall, using a competitive enzyme immunoassay (9). Culture media from wild-type and _gas1_Δ cells were assayed for the presence of β1,3-glucan. Medium of wild-type cells contained about 214 ng of β1,3-glucan/ml of culture, whereas culture medium of _gas1_Δ cells contained a fivefold-higher amount of β1,3-glucan (1,072 ng/ml). Culture medium from the _fks1_Δ mutant did not contain more β1,3-glucan than that from wild-type cells (234 ng/ml), indicating that the release of β1,3-glucan is specific for the _gas1_Δ mutant and suggesting that Gas1p is involved in cross-linking β1,3-glucan to the cell wall. Alternatively, one might speculate that the increase in β1,3-glucan in the medium of _gas1_Δ cells was caused by cell lysis as a consequence of increased fragility of _gas1_Δ cells. However, the slow-growth phenotype of _gas1_Δ cells could not be remediated by growing them on plates containing either 1 M sorbitol or 0.5 M KCl (data not shown). Furthermore, Western analysis of medium proteins from _gas1_Δ cells with anti-HDEL antiserum (kindly provided by N. Dean) did not reveal the presence of the luminal endoplasmic reticulum protein Kar2p (BiP) (data not shown). This indicates, again, that under these growth conditions, the _gas1_Δ mutant is not osmotically fragile.
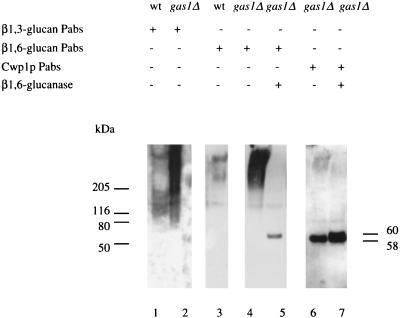
β-Glucosylated cell wall mannoproteins are covalently linked to the β1,3-glucan framework of the cell wall via β1,6-glucan (27, 37). Recently, it has been demonstrated definitively that β1,6-glucan is linked to a processed form of the GPI anchor of cell wall GPI proteins (31). If Gas1p is involved in the cross-linking of β1,3-glucan to the wall, one would predict that as a consequence of a defect in Gas1p activity, the incorporation of cell wall proteins would be affected as well. To test this, the culture media of wild-type and _gas1_Δ cells were analyzed for the presence of cell wall proteins. The culture medium of _gas1_Δ cells was found to contain three times more proteins than wild-type cells grown to the same cell density. Equal amounts of medium proteins, released from wild-type and _gas1_Δ cells, were separated by SDS-PAGE and blotted for Western analysis. The culture medium of _gas1_Δ cells contained high-molecular-weight proteins, reacting with both the anti-β1,3-glucan- and the anti-β1,6-glucan-specific antibodies (Fig. 1, lanes 2 and 4). In contrast, the culture medium of wild-type cells contained only a limited amount of β-glucosylated proteins reacting with either antiserum (Fig. 1, lanes 1 and 3).
FIG. 1.
_gas1_Δ cells secrete more β-glucosylated proteins into the medium. Western analysis of medium proteins from wild-type (lanes 1 and 3) and _gas1_Δ (lanes 2 and 4 through 7) cells was performed with the affinity-purified β1,3-glucan antiserum (lanes 1 and 2), the β1,6-glucan antiserum (lanes 3 through 5), or anti-Cwp1p antiserum (lanes 6 and 7). In each lane, 2.5 μg of protein was applied. The sizes of standard molecular mass markers are given on the left. wt, wild type; Pabs, polyclonal antibodies.
Treatment of the high-molecular-weight proteins in the culture medium of mutant cells with a purified endo-β1,6-glucanase (4) led to the disappearance of the β1,3-glucan epitope, indicating that the β1,3-glucan was attached to the proteins via β1,6-glucan (data not shown). Western analysis with the β1,6-glucan antiserum showed that the high-molecular-weight smear had disappeared. Instead, a prominent band of 60 kDa appeared (Fig. 1, lane 5). When the blot was stripped and reprobed with anti-Cwp1p antiserum, the 60-kDa band also reacted with the anti-Cwp1p antiserum (Fig. 1, lane 7). Apparently, the high-molecular-weight smear that disappeared after treatment with β1,6-glucanase contained β-glucosylated forms of Cwp1p. However, this smear reacted only weakly with the anti-Cwp1p antiserum (Fig. 1, lane 6), suggesting that the smear contained not only Cwp1p but possibly also other proteins. In addition, an abundant 58-kDa Cwp1p form which did not react with either the β1,6-glucan- or the β1,3-glucan-specific antibodies, and therefore did not contain β1,6-glucan and/or β1,3-glucan side chains, was detected in the mutant-cell culture medium (Fig. 1, lane 6; see also lanes 2 and 4). The 2-kDa difference in size between these two Cwp1p forms is probably due to β1,6-linked glucose residues remaining after enzymatic digestion. Only negligible amounts of Cwp1p were found in the culture medium of wild-type cells. From these data we conclude that disruption of GAS1 results in the release of β-glucosylated cell wall proteins, including Cwp1p, into the medium.
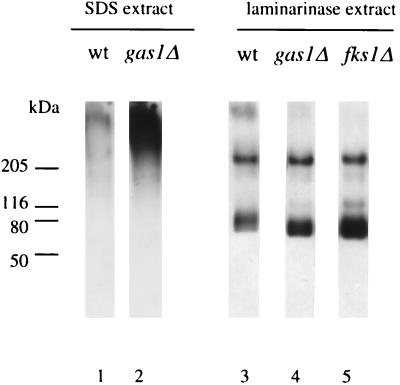
SDS-extractable mannoproteins appear to be noncovalently associated with the cell wall. It has been reported that the SDS-extractable forms of α-agglutinin protein from wild-type cells do not contain a β1,6-glucan epitope (33). In agreement with this, we could hardly detect β1,6-glucosylated proteins in the SDS extract of wild-type cell walls (Fig. 2, lane 1). Western analysis of SDS-extractable mannoproteins from a _gas1_Δ mutant with the β1,6-glucan antiserum revealed the presence of β1,6-glucosylated proteins, suggesting again that the incorporation of β1,6-glucosylated cell wall proteins was impaired (Fig. 2, lane 2). We next examined whether the deletion of GAS1 completely abolished the incorporation of β1,6-glucosylated cell wall proteins. To this end, SDS-extracted cell walls from wild-type and _gas1_Δ cells were digested with laminarinase (a mixture of β1,3- and β1,6-glucanase activities). Western analysis with β1,6-glucan-specific antibodies revealed that the cell walls of the _gas1_Δ mutant still contained β1,6-glucosylated cell wall mannoproteins. We recently demonstrated that the majority of the laminarinase-extracted β1,6-glucosylated mannoproteins in _gas1_Δ mutants were connected directly to chitin (28), a novel linkage identified by Kollár et al. (31). This was in contrast to the situation in wild-type cells, where most of the β1,6-glucosylated mannoproteins are linked to β1,3-glucan (28).
FIG. 2.
Western analysis of SDS- and laminarinase-released cell wall proteins of wild-type (wt) cells (lanes 1 and 3), _gas1_Δ cells (lanes 2 and 4), and _fks1_Δ cells (lane 5) using affinity-purified β1,6-glucan antiserum. Equal cell equivalents of SDS-released proteins (equivalent to 1 mg [fresh weight] of cell walls) and equal cell equivalents of laminarinase-released proteins (equivalent to 5 mg [fresh weight] of cell walls) were loaded.
Increased synthesis of specific cell wall components.
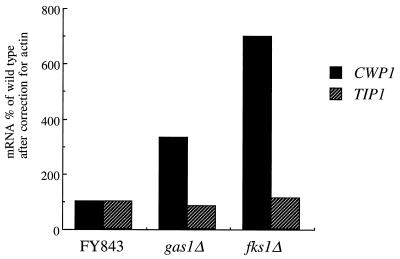
When laminarinase-released cell wall proteins are separated by SDS-PAGE and immunodetected with β1,6-glucan antibodies, a characteristic pattern of four β-glucosylated proteins with molecular sizes of approximately 60, 105, 135, and 240 kDa appears (27, 37, 53). It has further been shown that the 60-kDa band corresponds to Cwp1p and the 105-kDa band corresponds to Tip1p (27, 50, 53). The genes corresponding to the two other bands are still unknown. Western analysis with β1,6-glucan antibodies revealed that the amount of the 60-kDa protein in cell walls of the _gas1_Δ mutant was increased compared to that in wild-type cells (Fig. 2, lanes 3 and 4), whereas the amount of a second glucomannoprotein of about 240 kDa was comparable to the amount that is liberated from wild-type cell walls. The larger amount of laminarinase-extractable Cwp1p was not specific for the _gas1_Δ mutant and was also observed in the _fks1_Δ mutant (Fig. 2, lane 5). To determine whether the larger amounts of Cwp1p in the cell walls of _gas1_Δ and _fks1_Δ cells were due to a higher expression level of the protein or to more efficient release from the cell walls by laminarinase, the mRNA levels of CWP1 transcripts were determined in exponentially growing cells. Total RNAs from wild-type, _gas1_Δ, and _fks1_Δ cells grown on glucose were prepared and subjected to Northern analysis. Equal amounts of RNA (10 μg) were loaded. ACT1 mRNA levels were determined and used for normalization. Only up to 1.3-fold differences were found between the levels of ACT1 mRNA from different cell types, allowing the use of ACT1 mRNA for normalization (data not shown). The level of CWP1 mRNA was significantly higher in _gas1_Δ and _fks1_Δ mutant cells than in wild-type cells (Fig. 3). Thus, the larger amounts of laminarinase-extractable Cwp1p present in the cell walls of _gas1_Δ and _fks1_Δ cells are likely due to higher expression levels of this protein and not to more efficient extraction of this protein from the wall. The mRNA of another cell wall protein, Tip1p (53), was not affected, confirming that indeed the expression of CWP1 is induced in _gas1_Δ and _fks1_Δ cells (Fig. 3). These data indicate that deletion of GAS1 or FKS1 resulted in increased expression and incorporation of a specific cell wall protein, Cwp1p, into the cell wall. Whether the expression of other cell wall proteins is also induced remains to be determined.
FIG. 3.
_gas1_Δ and _fks1_Δ cells have higher transcript levels of CWP1 than wild-type cells. The amounts of mRNA of CWP1 and TIP1, both encoding cell wall mannoproteins, are shown as percentages of wild-type levels after correction for actin.
Analysis of the sugar compositions of the cell walls of _gas1/cwh52_Δ and _fks1/cwh53_Δ mutants showed a strong relative reduction of β-glucan compared to mannan and chitin (Table 1). This analysis, however, does not exclude the possibility that the amount of mannose had increased, because such an increase would also show up as an apparent reduction of cell wall β-glucan. To address this possibility, the absolute amounts of the different sugars per gram (dry weight) of entire cells were determined (Table 2). In wild-type cells, about 20% (dry weight) of the cell was sugars, in good agreement with earlier observations (14). Analysis of the _fks1_Δ mutant showed a 50% reduction in the amount of β-glucan, in agreement with its proposed function as a subunit of β1,3-glucan synthase (8, 21). The amounts of chitin and mannan had increased compared to those in wild-type cells. A similar analysis of the _gas1_Δ mutant showed a decrease of only 15% in cell wall β-glucan content. This was accompanied by an increase in the amounts of chitin and mannan. The higher levels of chitin and mannan in _gas1_Δ cells explained the previously observed large relative reduction in β-glucan. From this analysis we conclude that in both _fks1_Δ and _gas1_Δ mutant cells, the cell wall composition had changed. Both _fks1_Δ and _gas1_Δ mutants show decreases in β-glucan content, of 50 and 15%, respectively, and increases in chitin and mannan content.
TABLE 1.
Sugar compositions of isolated cell walls
| Strain or genotype | Sugar composition of cell wall (%)a | Glc/Man ratio (mean ± SEM)b | ||
|---|---|---|---|---|
| GN | Glc | Man | ||
| FY834 (wild type) | 1.0 | 51.1 | 47.9 | 1.07 ± 0.04 (n = 6) |
| cwh52-1 | 3.9 | 30.7 | 65.4 | 0.47 ± 0.03 (n = 2) |
| gas1/cwh52Δ | 2.3 | 36.0 | 61.7 | 0.58 ± 0.04 (n = 5) |
| cwh53-1 | 3.3 | 19.7 | 77.0 | 0.26 ± 0.01 (n = 2) |
| fks1/cwh53Δ | 3.7 | 20.8 | 75.5 | 0.28 ± 0.04 (n = 5) |
TABLE 2.
Sugar compositions of whole cells
| Strain | mg of sugara/g (dry wt) of whole cells (% of wild-type level) | Glc/Man ratio (mean ± SEM)b | |||
|---|---|---|---|---|---|
| GN | Glc | Man | ΣHexose | ||
| FY834 | 2.8 (100) | 76.6 (100) | 120.7 (100) | 200.1 (100) | 0.64 ± 0.02 |
| _gas1_Δ mutant | 4.7 (170) | 65.3 (85) | 167.5 (139) | 237.5 (119) | 0.39 ± 0.01 |
| _fks1_Δ mutant | 5.3 (190) | 38.5 (50) | 184.9 (153) | 228.7 (114) | 0.21 ± 0.01 |
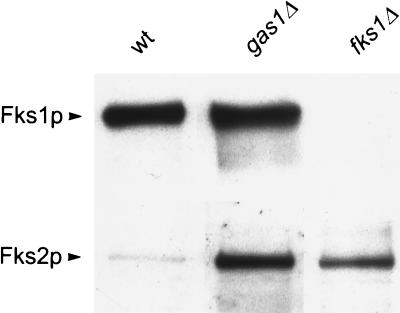
To exclude a direct negative effect of a gas1 deletion on the synthesis of β1,3-glucan, the in vitro β1,3-glucan synthase activity was determined. Whereas the activities found in wild-type cells (423 nmol h−1 mg of protein−1) and _fks1_Δ cells (66 nmol h−1 mg of protein−1) were as expected and comparable to previously found values (35), the β1,3-glucan synthase activity found in _gas1_Δ cells (586 nmol h−1 mg of protein−1) was actually elevated. Western analysis using Fks1p- and Fks2p-specific antibodies showed that both Fks1p and Fks2p were expressed in _gas1_Δ cells, in contrast to wild-type cells, where only Fks1p was expressed under these conditions (Fig. 4). The simultaneous expression of Fks1p and Fks2p is consistent with the increase in β1,3-glucan synthase activity found in the _gas1_Δ mutant. It has been reported previously that the residual β1,3-glucan synthase activity in the _fks1_Δ mutant is caused by the expression of Fks2p, under conditions where normally FKS2 is not expressed. It is therefore possible that in both the _gas1_Δ mutants and the _fks1_Δ mutants, expression of FKS2 is specifically induced as a response to their weakened cell walls.
FIG. 4.
Expression of Fks2p is elevated in _gas1_Δ cells. Shown are results of Western blot analysis of Fks1p and Fks2p in strains FY834 (wild type [wt]), AR104 (_gas1_Δ), and AR100 (_fks1_Δ) grown in YPD medium. Membrane samples (20 μg of protein) from the indicated strains were subjected to SDS-PAGE and Western blot analysis. Blots were separately probed with anti-Fks1p and anti-Fks2p antisera as previously described (35, 36).
DISCUSSION
In this study we have searched for a function of Gas1p. Mutations in FKS1 and GAS1 resulted in a lower glucan content of the cell wall (Tables 1 and 2). Furthermore, _fks1_Δ and _gas1_Δ cells became larger and more spherical than wild-type cells and hypersensitive to Calcofluor White (42, 46). In contrast to _fks1_Δ cells, however, the β1,3-glucan synthase activity in _gas1_Δ cells was not decreased but actually slightly increased. These phenotypes suggest that Gas1p is essential for normal cell wall construction, but they seem to exclude a role for Gas1p in the formation of β1,3-glucan. We therefore examined whether Gas1p might be involved in retaining β1,3-glucan in the cell wall. The culture medium of the _gas1_Δ mutant contained five times more β1,3-glucan than that of wild-type cells, and this is probably even an underestimate in view of the presence of exo-β1,3-glucanases in the medium, which probably degrade the released glucan. The culture medium of the _fks1_Δ mutant did not contain more β1,3-glucan than that of wild-type cells, indicating that the release of β1,3-glucan is specific for _gas1_Δ cells. These results suggest that Gas1p is involved in a cell wall assembly or remodeling step involving β1,3-glucan, possibly functioning as a transglucosylating enzyme.
β1,3-Glucan is known to be linked to chitin (30). It seems, however, less likely that Gas1p is involved in cross-linking chitin and β1,3-glucan, because the number of cross-links between chitin and β1,3-glucan was not significantly affected in a _gas1_Δ mutant (29a). β1,3-Glucan is also linked to cell wall proteins through β1,6-glucan (27). It has further been shown that mutants deficient in β1,6-glucan synthesis are defective in the incorporation of cell wall proteins such as Cwp1p and α-agglutinin (23, 33). Thus, the attachment of β1,6-glucan to cell wall mannoproteins seems to be required for proper incorporation in the cell wall. Gas1p seems not to be involved in the attachment of β1,6-glucan to cell wall proteins, since the cell wall proteins found in the medium of a _gas1_Δ mutant do contain β1,6-glucan (Fig. 1, lane 4). In addition, these proteins also carry β1,3-glucan (Fig. 1, lane 2), which makes it less likely that Gas1p is responsible for linking β1,3-glucan to protein-bound β1,6-glucan. This raises the question whether Gas1p might assist in a later, as yet undefined step, which finally anchors cell wall proteins, for example, by molecular remodeling of the protein-bound β1,3-glucan–β1,6-glucan heteropolymer.
Further characterization of the _gas1_Δ mutant indicated that in order to compensate for the loss of the function of Gas1p and its corresponding weakening of the cell wall, _gas1_Δ cells seem to respond by changing the composition and structure of the cell wall. Carbohydrate analysis of _gas1_Δ mutants showed that the amounts of chitin and mannan had increased compared to those in wild-type cells. The increase in chitin content in a _gas1_Δ mutant has also been reported by Popolo et al. (43). This phenotype, a higher mannan and chitin content, was not specific for _gas1_Δ mutants and was also found in cell walls of fks1 mutants (Table 2). A higher chitin content has also been observed in the knr4 mutant, in which, as in the fks1 mutant, the synthesis of β1,3-glucan is affected (20). The higher chitin content in the cell walls of these mutants might therefore be a general response to cell wall defects, to compensate for the loss in strength of the cell wall. Cells with mutations in either fks1, gas1, or knr1 tend to swell and become more spherical, suggesting that as a result of their weakened cell walls, hypo-osmotic stress-like conditions are created. This phenomenon is probably not limited to β1,3-glucan mutants, since other osmolabile mutants, such as mutants affected in N- and O-glycosylation, also have increased levels of chitin in the wall (16, 45). Interestingly, in several fungi that were subjected to hypo-osmotic stress, increased chitin synthase activities have been found (17).
Another phenotype observed in _knr4_Δ, _gas1_Δ, and _fks1_Δ mutants was their increased resistance to β1,3-glucanase treatments (19, 42, 44a), indicating a change in cell wall architecture. This might be explained by the increased number of linkages between chitin and β1,6-glucosylated cell wall mannoproteins (28), making the walls rather insensitive to β1,3-glucanases. The observed resistance to β1,3-glucanase might also be partially caused by the higher Cwp1p content of the cell wall, since glucanase-extractable mannoproteins, like Cwp1p, determine the permeability of the cell wall for macromolecules (6).
The higher mannan content might be due to a higher expression of cell wall mannoproteins. We found increases in the amounts of Cwp1p present in cell walls of _gas1_Δ and _fks1_Δ cells compared to those in wild-type cells (Fig. 2, lanes 3 to 5). The larger amounts of Cwp1p were in good agreement with the higher levels of CWP1 mRNA in these mutants (Fig. 3). The higher levels of Cwp1p might, therefore, like the increase in chitin content, be part of a general response of the cell to cell wall defects in an attempt to prevent cell lysis.
A third phenotype observed in the _gas1_Δ mutant that might be involved in compensating for a loss in cell wall integrity is the increase in β1,3-glucan synthase activity. Western analysis showed that the increase in β1,3-glucan synthase activity is probably due to the simultaneous expression of Fks1p and Fks2p genes, both encoding putative subunits of the β1,3-glucan synthase complex (35). In vegetative, glucose-grown wild-type cells, usually only Fks1p is expressed. Like the increased chitin synthesis and the induction of Cwp1p expression, the elevated expression of Fks2p also occurred in _fks1_Δ cells.
The completion of the S. cerevisiae genome has identified a family of five GAS genes (GAS1 through GAS5; open reading frames YMR307w, YLR343w, YMR215w, YOL132w, and YOL30w, respectively). The amino acid identities between the different genes are 32 to 51%. All five members of this gene family contain an N-terminal signal sequence for entrance into the endoplasmic reticulum and a putative C-terminal signal sequence for the addition of a GPI anchor. Only disruption of GAS1 resulted in a Calcofluor White-hypersensitive phenotype. Disruption of any other single GAS gene did not result in an apparent growth defect. Mutants in which multiple GAS genes are disrupted, including the mutant in which all five homologs have been disrupted, are still viable and do not show additional phenotypes compared to the gas1 single mutant (44a). Experiments addressing the expression and the function of the different GAS homologs await further study.
Taken together, our results indicate that in mutants with weakened cell walls, the cell is able to activate a cell wall repair mechanism, probably to ensure cell integrity. This raises the question of how this signal is generated. In S. cerevisiae, a protein kinase cascade called the PKC1 pathway plays a central role in maintaining cell wall integrity. A general mechanism for activation of the PKC1 pathway has been proposed by Kamada et al. (24). In this model, weakening of the cell wall is detected as plasma membrane stretch, possibly via mechanosensitive channels, which results in the activation of the PKC1 pathway. It is tempting to speculate that in mutants with weakened cell walls, cells are under constant membrane stretch, and that this activates the PKC1 pathway, thereby inducing several compensatory reactions. Experimental support for this hypothesis comes from the observations that _fks1Δ pkc1_Δ and _gas1Δ pkc1_Δ double mutants are synthetic lethal (15, 43) and that thermal induction of FKS2 is Pkc1p dependent (25).
ACKNOWLEDGMENTS
We thank Roman Kollár and E. Cabib for their chitin–β1,3-glucan linkage analysis in the _gas1_Δ mutant, J. Vossen for the _cwp1_Δ deletion strain, N. Dean for the anti-HDEL antiserum, M. van der Vaart for the YDp plasmids, H. Shimoi for the anti-Cwp1p antibodies, and J. de la Cruz and A. Llobell for the purified endo-β1,6-glucanase.
J.C.K. acknowledges the financial support of the Netherlands Technology Foundation (STW).
REFERENCES
- 1.Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K. Current protocols in molecular biology (CD-ROM). New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 1a.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Culin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berben G J, Dumont J, Gilliquet V, Bolle P, Hilger F. The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast. 1991;7:475–477. doi: 10.1002/yea.320070506. [DOI] [PubMed] [Google Scholar]
- 3.Castro C, Ribas J C, Valdivieso H, Varona R, del Rey F, Duran A. Papulacandin B resistance in budding and fission yeasts: isolation and characterization of a gene involved in (1,3)β-d-glucan synthesis in Saccharomyces cerevisiae. J Bacteriol. 1995;177:5732–5739. doi: 10.1128/jb.177.20.5732-5739.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Cruz J, Pintor-Toro J A, Benítez T, Llobell A. Purification and characterization of an endo-β-1,6-glucanase from Trichoderma harzianum that is related to its mycoparasitism. J Bacteriol. 1995;177:1864–1871. doi: 10.1128/jb.177.7.1864-1871.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Nobel J G, Dijkers C, Hooijenberg E, Klis F M. Increased cell wall porosity in Saccharomyces cerevisiae after treatment with dithiothreitol or EDTA. J Gen Microbiol. 1989;135:2077–2084. [Google Scholar]
- 6.De Nobel J G, Klis F M, Priem J, Munnik T, Van Den Ende H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast. 1990;6:491–499. doi: 10.1002/yea.320060606. [DOI] [PubMed] [Google Scholar]
- 7.Douglas C M, Marrinan J A, Li W, Kurtz M B. A Saccharomyces cerevisiae mutant with echinocandin-resistant 1,3-β-d-glucan synthase. J Bacteriol. 1994;176:5686–5696. doi: 10.1128/jb.176.18.5686-5696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas C M, Foor F, Marrinan J A, Morin N, Nielsen J B, Dahl A M, Mazur P, Baginsky W, Li W, El-Sherbeini M, Clemas J A, Mandale S M, Frommer B R, Kurtz M B. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-d-glucan synthase. Proc Natl Acad Sci USA. 1994;91:12907–12911. doi: 10.1073/pnas.91.26.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douwes J, Doukes G, Montijn R C, Heederik D, Brunekreef B. Measurement of β(1→3)-glucans in occupational and home environments with an inhibition enzyme immunoassay. Appl Environ Microbiol. 1996;62:3176–3182. doi: 10.1128/aem.62.9.3176-3182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drgonová J, Drgon T, Tanaka K, Kollár R, Chen G-C, Ford R A, Chan C S M, Takai Y, Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996;272:277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- 11.Eng W, Faucette L, McLaughlin M M, Cafferkey R, Koltin Y, Morris R A, Young P R, Johnson R K, Livi G P. The yeast FKS1 gene encodes a novel membrane protein, mutations in which confer FK506 and cyclosporin A hypersensitivity and calcineurin dependent growth. Gene. 1994;151:61–71. doi: 10.1016/0378-1119(94)90633-5. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg A P, Vogelstein B. A technique for radiolabeling DNA restriction fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 13.Fleet G H, Manners D J. Isolation and composition of an alkali-soluble glucan from the cell walls of Saccharomyces cerevisiae. J Gen Microbiol. 1976;94:180–192. doi: 10.1099/00221287-94-1-180. [DOI] [PubMed] [Google Scholar]
- 14.Fleet G H. Cell walls. In: Rose A H, Harrison J S, editors. The yeasts. Vol. 4. New York, N.Y: Academic Press, Inc.; 1991. pp. 199–277. [Google Scholar]
- 15.Garrett-Engele P, Moilanen B, Cyert M S. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H+-ATPase. Mol Cell Biol. 1995;15:4103–4114. doi: 10.1128/mcb.15.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentzsch M, Tanner W. The PMT gene family: protein O-glycosylation in Saccharomyces cerevisiae is vital. EMBO J. 1996;15:5752–5759. [PMC free article] [PubMed] [Google Scholar]
- 17.Gooday G W, Schofield D A. Regulation of chitin synthesis during growth of fungal hyphae: the possible participation of membrane stress. Can J Bot. 1995;73:114–121. [Google Scholar]
- 18.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of E. coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 19.Hong Z, Mann P, Brown N H, Tran L E, Shaw K J, Hare R S, DiDomenico B. Cloning and characterization of KNR4, a yeast gene involved in (1,3)-β-glucan synthesis. Mol Cell Biol. 1994;14:1017–1025. doi: 10.1128/mcb.14.2.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong Z, Mann P, Shaw K J, DiDomenico B. Analysis of β-glucans and chitin in a Saccharomyces cerevisiae cell wall mutant using high-performance liquid chromatography. Yeast. 1994;10:1083–1092. doi: 10.1002/yea.320100810. [DOI] [PubMed] [Google Scholar]
- 21.Inoue S B, Takewaki N, Takasuka T, Mio T, Adachi M, Fujii J, Miyamoto C, Arisawa M, Furuichi Y, Watanabe T. Characterization and gene cloning of 1,3-β-d-glucan synthase from Saccharomyces cerevisiae. Eur J Biochem. 1995;321:845–854. doi: 10.1111/j.1432-1033.1995.tb20770.x. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Fukuda Y, Murata M, Kimura A. Transformations of intact yeast cells with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang B, Sheraton J, Ram A F J, Dijkgraaf G J P, Klis F M, Bussey H. CWH41 encodes a novel endoplasmic reticulum membrane N-glycoprotein involved in β1,6-glucan assembly. J Bacteriol. 1996;178:1162–1171. doi: 10.1128/jb.178.4.1162-1171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamada Y, Jung U S, Piotrowski J, Levin D E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- 25.Kamada Y, Quadota H, Python C P, Anraku Y, Ohya Y, Levin D E. Activation of yeast protein kinase C by Rho1 GTPase. J Biol Chem. 1996;271:9193–9196. doi: 10.1074/jbc.271.16.9193. [DOI] [PubMed] [Google Scholar]
- 26.Kapteyn J C, Montijn R C, Dijkgraaf G J P, Van Den Ende H, Klis F M. Covalent association of β-1,3-glucan with β-1,6-glucosylated mannoproteins in cell walls of Candida albicans. J Bacteriol. 1995;177:3788–3792. doi: 10.1128/jb.177.13.3788-3792.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapteyn J C, Montijn R C, Vink E, De La Cruz J, Llobell A, Douwes J E, Shimoi H, Lipke P N, Klis F M. Retention of Saccharomyces cerevisiae cell wall proteins through a phosphodiester-linked β1,3-/β-1,6-glucan heteropolymer. Glycobiology. 1996;6:337–345. doi: 10.1093/glycob/6.3.337. [DOI] [PubMed] [Google Scholar]
- 28.Kapteyn J C, Ram A F J, Groos E M, Kollár R, Montijn R C, Van Den Ende H, Llobell A, Cabib E, Klis F M. Altered extent of cross-linking of β1,6-glucosylated mannoproteins to chitin in Saccharomyces cerevisiae mutants with reduced cell wall β1,3-glucan content. J Bacteriol. 1997;179:6279–6284. doi: 10.1128/jb.179.20.6279-6284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klis F M. Review: cell wall assembly in yeast. Yeast. 1994;10:851–869. doi: 10.1002/yea.320100702. [DOI] [PubMed] [Google Scholar]
- 29a.Kollár, R., and E. Cabib. Personal communication.
- 30.Kollár R, Petrakova E, Ashwell G, Robbins P W, Cabib E. Architecture of the yeast cell wall: the linkage between chitin and β(1,3)-glucan. J Biol Chem. 1995;270:1170–1178. doi: 10.1074/jbc.270.3.1170. [DOI] [PubMed] [Google Scholar]
- 31.Kollár R, Reinhold B B, Petrakova E, Yeh H J C, Ashwell G, Drgonova J, Kapteyn J C, Klis F M, Cabib E. Architecture of the yeast cell wall: β(1,6)-glucan interconnects mannoproteins, β(1,3)-glucan, and chitin. J Biol Chem. 1997;272:17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Lu C-F, Montijn R C, Brown J L, Klis F M, Kurjan J, Bussey H, Lipke P N. Glycosylphosphatidylinositol-dependent cross-linking of α-agglutinin and β-1,6-glucan in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1995;128:333–340. doi: 10.1083/jcb.128.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manners D J, Masson A J, Patterson J C. The structure of a β-1-3-d-glucan from yeast cell walls. Biochem J. 1973;135:19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazur P, Morin N, Baginsky W, El-Sherbeini M, Clemas J A, Nielsen J B, Foor F. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol Cell Biol. 1995;15:5671–5681. doi: 10.1128/mcb.15.10.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazur P, Baginsky W. In vitro activity of 1,3-β-d-glucan synthase requires the GTP-binding protein Rho1. J Biol Chem. 1996;217:14604–14609. doi: 10.1074/jbc.271.24.14604. [DOI] [PubMed] [Google Scholar]
- 37.Montijn R C, Van Rinsum J, Van Schagen F A, Klis F M. Glucomannoproteins in the cell wall of Saccharomyces cerevisiae contain a novel type of carbohydrate side chain. J Biol Chem. 1994;269:19338–19342. [PubMed] [Google Scholar]
- 38.Nuoffer C, Jeno P, Conzelmann A, Riezman H. Determinants for glycophospholipid anchoring of the Saccharomyces cerevisiae GAS1 protein to the plasma membrane. Mol Cell Biol. 1991;11:27–37. doi: 10.1128/mcb.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orlean P. Biogenesis of yeast wall and surface components. In: Pringle J R, Broach J R, Jones E W, editors. Molecular and cellular biology of the yeast Saccharomyces. 3. Cell cycle and cell biology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 229–362. [Google Scholar]
- 40.Ozols J. Amino acid analysis. Methods Enzymol. 1990;182:587–601. doi: 10.1016/0076-6879(90)82046-5. [DOI] [PubMed] [Google Scholar]
- 41.Parent S A, Nielsen J B, Morin N, Chrebet G, Ramadan N, Dahl A M, Hsu M, Bostian K A, Foor F. Calcineurin-dependent growth of an FK506- and CsA-hypersensitive mutant of S. cerevisiae. J Gen Microbiol. 1993;139:2973–2984. doi: 10.1099/00221287-139-12-2973. [DOI] [PubMed] [Google Scholar]
- 42.Popolo L, Vai M, Gatti E, Porello S, Bonfante P, Balestrini R, Alberghina L. Physiological analysis of mutants indicates involvement of the Saccharomyces cerevisiae GPI-anchored protein gp115 in morphogenesis and cell separation. J Bacteriol. 1993;175:1879–1885. doi: 10.1128/jb.175.7.1879-1885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popolo L, Gilardelli D, Bonfante P, Vai M. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1Δ mutant of Saccharomyces cerevisiae. J Bacteriol. 1997;179:463–469. doi: 10.1128/jb.179.2.463-469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quadota H, Python C P, Inoue S B, Arisawa M, Ankaru Y, Zheng Y, Watanabe T, Levin D E, Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science. 1996;272:279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
- 44a.Ram, A. Unpublished data.
- 45.Ram A F J, Wolters A, ten Hoopen R, Klis F M. A new approach for isolating cell wall mutants in Saccharomyces cerevisiae by screening for hypersensitivity to Calcofluor White. Yeast. 1994;10:1019–1030. doi: 10.1002/yea.320100804. [DOI] [PubMed] [Google Scholar]
- 46.Ram A F J, Brekelmans S S C, Oehlen L J W M, Klis F M. Identification of two cell cycle regulated genes affecting the β1,3-glucan content of cell walls in Saccharomyces cerevisiae. FEBS Lett. 1995;358:165–170. doi: 10.1016/0014-5793(94)01418-z. [DOI] [PubMed] [Google Scholar]
- 47.Rose M D, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 49.Schreuder M P, Brekelmans S S C, Van Den Ende H, Klis F M. Targeting of a heterologous protein to the cell wall of Saccharomyces cerevisiae. Yeast. 1993;9:399–409. doi: 10.1002/yea.320090410. [DOI] [PubMed] [Google Scholar]
- 50.Shimoi H, Iimura Y, Obata T. Molecular cloning of CWP1: a gene encoding a Saccharomyces cerevisiae cell wall protein solubilized with Rarobacter faecitabidus protease I. J Biochem. 1995;118:302–311. doi: 10.1093/oxfordjournals.jbchem.a124907. [DOI] [PubMed] [Google Scholar]
- 51.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 52.Vai M, Gatti E, Lacana E, Popolo L, Alberghina L. Isolation and deduced amino acid sequence of the gene encoding gp115, a yeast glycophospholipid-anchored protein containing a serine-rich region. J Biol Chem. 1991;266:12242–12248. [PubMed] [Google Scholar]
- 53.Van Der Vaart J M, Caro L H P, Chapman J W, Klis F M, Verrips C T. Identification of three mannoproteins in the cell wall of Saccharomyces cerevisiae. J Bacteriol. 1995;177:3104–3110. doi: 10.1128/jb.177.11.3104-3110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winston F, Dollard C, Ricupero S L. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]