Immunologic function and survival in hemodialysis patients (original) (raw)
. Author manuscript; available in PMC: 2018 Sep 20.
Abstract
Background.
Although the medical determinants of mortality in patients with end-stage renal disease (ESRD) treated with hemodialysis (HD) are well appreciated, the contribution of immunologic parameters to survival in such patients is unclear, especially when variations in age, medical comorbidity and nutrition are controlled. In addition, although dysregulation of cytokine metabolisn has been appreciated in patients with ESRD, the association of these parameters with outcomes has not been established. Recently, the type of dialyzer used in patients’ treatment has been associated with survival, but the mechanisms underlying these findings, including their immune effects, have not been established. We conducted a prospective, cross-sectional, observational multicenter study of urban HD patients to determine the contribution of immunological factors to patient survival. We hypothesized increased proinflammatory cytokines would be associated with increased mortality, and that improved immune function would be associated with survival.
Methods.
Patients were assessed using demographic and anthropometric indices, Kt/V, protein catabolic rate (PCR) and immunologic variables including circulating cytokine [interleukin (IL)-1, IL-2, IL-4, IL-5, IL-6, IL-12, IL-13 and tumor necrosis factor (TNF)-_α_] levels, total hemolytic complement activity (CH50), and T cell number and function. A severity index, previously demonstrated to be a mortality marker, was used to grade medical comorbidity. A Cox proportional hazards model, controlling for patients’ age, severity index, level of serum albumin concentration, dialyzer type and dialysis site was used to assess relative survival risk.
Results.
Two hundred and thirty patients entered the study. The mean (± sd) age of the population was 54.4 ± 14.2 years, mean serum albumin concentration was 3.86 ± 0.47 g/dl, mean PCR was 1.1 ± 0.28 g/kg/day, and mean Kt/V 1.2 ± 0.3. Patients’ serum albumin concentration was correlated with levels of Kt/V and PCR, and their circulating IL-13 and TNF-α levels, but negatively with their circulating IL-2 levels, T-cell number and T-cell antigen recall function. T-cell antigen recall function correlated negatively with PCR, but not Kt/V. There was no correlation of any other immune parameter and medical or demographic factor. Immune parameters, however, were all highly intercorrelated. Mean level of circulating cytokines in HD patients were in all cases greater than those of a normal control group. There were few differences in medical risk factors or immune parameters between patients treated with different types of dialyzers. After an almost three-year mean follow-up period, increased IL-1, TNF-α, IL-6, and IL-13 levels were significantly associated with increased relative mortality risk, while higher levels of IL-2, IL-4, IL-5, IL-12, T-cell number and function, and CH50 were associated with improved survival. The difference in survival between patients treated with unmodified cellulose dialyzers and modified or synthetic dialyzers approached the level of statistical significance, but there were no differences in levels of circulating cytokines between these two groups.
Conclusions.
Higher levels of circulating proinflammatory cytokines are associated with mortality, while immune parameters reflecting improved T-cell function are associated with survival in ESRD patients treated with HD, independent of other medical risk factors. These factors may serve as markers for outcome. The mechanism underlying the relationship of immune function and survival, and the effect of interventions to normalize immune function in HD patients should be studied.
Keywords: cytokine, interleukin, tumor necrosis factor-α, T-cell, survival, dialyzer, chronic kidney failure, hemodialysis
The medical determinants of mortality in patients with end-stage renal disease (ESRD) treated with hemodialysis (HD) consist of older age, presence of diabetes mellitus, and to a lesser extent, of comorbid conditions such as cardiovascular and cerebrovascular disease, cancer, collagen vascular disease and chronic obstructive pulmonary disease [1]. For the United States’ ESRD population, African-American and women patients tend to have better survival [1]. The role of nutrition [2, 3] and dose of dialysis administered (Kt/V) [2–6] in improving survival in HD patients has been increasingly appreciated. Recently, a study has suggested the type of dialyzer used for chronic patient treatment may have an impact on patients’ survival [7]. The mechanism underlying this effect has been poorly understood.
Several studies have suggested various aspects of immune dysregulation in HD patients [8–11], but few studies have linked these factors to medical parameters, or with outcome. We hypothesized higher levels of circulating proinflammatory cytokines would be associated with mortality, while immune parameters associated with improved T-cell function would be associated with survival in ESRD patients treated with HD, independent of other medical risk factors. In order to test these hypotheses, we prospectively studied the interrelationship between medical and nutritional factors, dose of dialysis, and immunologic function in patients with ESRD treated with HD.
METHODS
Patient population and demographics of the study hemodialysis units
Patient recruitment began September 1, 1992 and concluded March 31, 1996. The observation period ended August 31, 1996. George Washington University Medical Center’s Ambulatory Dialysis Unit (GWUMC), Howard University Medical Center’s Dialysis Unit (HUMC), and the Washington Veterans Affairs Medical Center Dialysis Unit (VAMC), all in Washington D.C., were the study sites. The population of the three units was primarily composed of African-American patients. All patients enrolled in chronic ESRD HD programs at the GWUMC, HUMC and VAMC dialysis units, with the exception of HIV infected patients, hospitalized patients, patients who had a psychiatric diagnosis of psychosis and patients who scored less than 23 on a mini-mental status exam [12] were eligible. Patients were assigned to treatment with diffferent types of dialyzers at the different centers by ten different nephrologists, according to unit policy, personal preference and clinical considerations. Written informed consent was obtained at GWUMC and HUMC, while verbal consent was obtained prior to each patient’s enrollment at the VAMC. The study was approved by the institutional review boards of the three medical centers. Twenty-eight healthy subjects, not taking medications, were recruited as controls for the evaluations of circulating cytokine levels, after being assessed with normal physical and screening laboratory examinations. All subjects had normal blood pressure, normal levels of blood urea nitrogen and serum creatinine concentration, and normal urinalyses.
Medical risk factors
Disease severity (the product of age and the relative mortality risk of comorbid conditions such as diabetes mellitus, cardiovascular and cerebrovascular disease, collagen vascular disease, malignancy and the type of renal disease) was quantified by the ESRD severity coefficient [13], previously validated by a survival analysis in a large sample of ESRD patients.
Nutritional and dialytic parameters
Protein catabolic rate (PCR) and Kt/V were calculated monthly at GWUMC and HUMC, and quarterly at VAMC using the percent urea reduction method as outlined by Jindal, Manuel and Goldstein [14] and used previously in other studies [2]. The mean of the patients’ three sequential monthly serum albumin concentrations after enrollment was determined. Mid-arm circumference and arm muscle area were obtained by trained personnel at study entry, as previously described [15–17]. Dialyzer type was not determined by the study protocol, but was noted for each patient at the time of assesssment of immune variables and categorized as (1) unmodified cellulose, (2) modified cellulose or (3) synthetic [7].
Immunologic variables
Immunologic variables were assessed in patients in the three dialysis units at the time of their regular monthly laboratory evaluations. Blood samples obtained before initiating HD were immediately processed for recovery of plasma and lymphocytes. Briefly, plasma was removed, aliquoted, and stored at −70°C. The buffy coat was carefully removed, layered on a Ficol-Hypaque (Sigma Chemical Co., St. Louis, MO, USA) gradient and spun at 800 g for 12 minutes.
Evaluation of cell mediated immunity was performed by subtyping lymphocytes using fluorescein-labeled antibodies (Anti-CD3, -CD4, -CD8, or -CD19; PharmMinGen, San Diego, CA, USA). Additionally, T-cell functional studies [18, 19] were performed by coincubating the isolated lymphocytes with a panel of common antigens (purified protein derivative, streptolysin, candida extract, and tetanus toxoid; Allergenic, Mountain View, CA, USA). The cells were washed prior to the addition of 0.2 _μ_Ci of tritiated thymidine to each tube, then pulsed for 18 hours at 37°C, washed in medium and harvested. The incorporated radioactivity present in each cell pellet was measured in a Beckman LS3801 scintillation counter (Beckman Instruments, Fullerton, CA, USA) and the mean counts of triplicate samples expressed as an antigen recall stimulation index, using the following formula:
Stimulation index=Average cpm of each triplicate sampleAverage cpm of the media controls
Total complement activity (CH50) was measured using a hemolytic assay (Kallestad, Austin, TX, USA) [20]. Circulating cytokine [interleukin (IL)-1, IL-2, IL-4, IL-5, IL-6, IL-12, IL-13, and tumor necrosis factor (TNF)-_α_] concentrations were detected and measured by a chemiluminescence-enhanced capture ELISA modification [20, 21] of the technique described by Thorpe et al [22] using specific antibodies directed against each cytokine (R & D Systems, Minneapolis, MN and Bachem Biosciences, Inc., King of Prussia, PA, USA) of interest.
Statistical methods
Stability of immunological variables was assessed by correlating individuals’ T-cell numerical and functional, cytokine levels at study entry, and at a second assessment 90 to 270 days thereafter in 163 of the 230 subjects, as previously described [17]. All of the circulating cytokines displayed positively skewed distributions. Log transformations produced relatively symmetric distributions in all cases. Correlations between demographic data, medical risk factors, and immunologic factors were assessed by Spearman Rank Order correlation coefficients, because of the skewed distributions of the immunologic data, as previously described [17]. Because of the number of comparisons assessed, P = 0.01 was taken as the level of statistical significance for correlations. Differences between groups were assessed by unpaired _t_-tests, the Wilcoxon test for comparison of data not distributed normally, chi-square analysis and analysis of variance (ANOVA) as appropriate.
Survival time for each individual patient was determined by the number of days between the initial study evaluation and the end of the study observation period or date of death. Survival status was confirmed using the Health Care Finance Administration data base, obtained through ESRD Network 5 (Richmond, VA, USA) for all patients enrolled in the study. Cox proportional hazards regression was used to predict mortality hazard [23]. A separate equation was calculated for each medical and demographic predictor (age at time of entry into the study, gender, severity coefficient, serum albumin concentration, dialyzer type, and Kt/V determined at study entry).
Following the results of these initial bivariate Cox regressions, regression analyses were performed in which the relationship between immunologic variables and survival were examined while simultaneously controlling for predictor medical risk factors, specifically patients’ age, severity coefficient, level of serum albumin concentration, dialyzer type and dialysis site. Relative risks or hazards represent the expected change in mortality risk associated with a 1 sd increase in the predictor variable, except for dialysis site, dialyzer type and gender, or as otherwise noted. This allows for comparison of the effects of changes in levels of several risk factors across different parameters in a population [24]. For log transformed variables, the standardized relative risk represents the increased risk associated with a 100% increase (or doubling) of the untransformed predictor variable. Analyses were performed using PROC PHREG in SAS 6.12 (SAS Institue Inc., Cary, NC, USA) using the exact method for ties. The alpha level of tests of survival and group differences was 0.05. Data are presented as mean ± sd.
RESULTS
Demographics
The total enrolled sample comprised 230 subjects (Table 1), representing an enrollment rate of 56.8% of eligible subjects in the three units. Ninety-three percent of our patient population was comprised of African-Americans; 30.9% were female, and 57.8% had diabetes mellitus. The mean age of our patient population was 54.4 ± 14.2 years. The patients had received renal replacement therapy for a mean and median of 33.7 ± 47.3 months and 15.7 months, respectively, at the time of enrollment. The patients’ mean PCR was 1.1 ± 0.28 g/kg/day, mean Kt/V was 1.2 ± 0.3, and mean serum albumin concentration was 3.86 ± 0.47 g/dl. The mean arm muscle area and upper arm circumference in our study population was 545.6 ± 190 cm2, and 30.3 ± 6.2 cm, respectively. Mean and median follow-up times were 35.4 ± 8.3 and 38.4 months, respectively. All the healthy controls were African-Americans. The mean age of the 11 male and 17 female healthy controls was 52.1 ± 5.5 years. There was no difference in mean age between the men and the women in the control group.
Table 1.
Patient demographics of the study
| Site | N | Male | Female | White | Black | Hispanic |
|---|---|---|---|---|---|---|
| GWU | 78 | 49 | 29 | 12 | 65 | 1 |
| HUH | 89 | 50 | 39 | 0 | 89 | 0 |
| VAMC | 63 | 60 | 3 | 3 | 60 | 0 |
| Total | 230 | 159 | 71 | 15 | 214 | 1 |
Characteristics of patients treated with different dialyzer types
Regarding dialyzer types, 41.3% of patients were treated with unmodified cellulose, 14.3% with modified cellulose and 44.3% with synthetic dialyzers. 92.1% of patients at the VAMC, 55.1% of patients at GWUMC, and 1.1% of patients at HUMC were treated with synthetic dialyzers. The distribution of patients treated with unmodified cellulose dialyzers was: 75.3% of patients at HUMC, 7.9% of patients at the VAMC, and 29.5% of patients at GWUMC. There was no difference between the mean age or the proportion of patients with diabetes mellitus treated with each type of dialyzer (ANOVA). Fewer women were treated with synthetic, and more were treated with unmodified cellulose dialyzers, in part because of the lower proportion of women treated at the VAMC (ANOVA). The mean serum albumin concentration was lower in patients treated with modified cellulose compared with unmodified cellulose and synthetic dialyzers (3.54 ± 0.51 vs. 3.88 ± 0.47, and 3.93 ± 0.41 g/dl, P < 0.0001, ANOVA). Mean Kt/V was not significantly different in patients treated with synthetic (1.28 ± 0.35), unmodified (1.19 ± 0.28) or modified cellulose dialyzers (1.24 ± 0.33, ANOVA).
Immune parameters
Intrassay and interassay coefficients of variation for circulating cytokine measurements (15 repetitive samples performed on five separate days) ranged between 2.18 and 4.59%. There was no difference in mean level of circulating cytokines between the men and the women in the control group. The mean level of all the circulating cytokines was lower in the healthy control group compared with the HD patients (Table 2). There was no difference in the mean level of any assessed immunologic parameter between the three groups of patients treated with different dialyzers, with the exception of T-cell recall function (greater in the group treated with modified cellulose dialyzers, compared with unmodified cellulose and synthetic dialyzers: 22.8 ± 10 vs. 20 ± 11.2, 17.2 ± 11.2 SI units, P < 0.035, ANOVA). There was no difference in mean levels of assessed immunologic parameters in patients with and without diabetes mellitus. Patients’ individual immune parameters at first assessment and thereafter all also correlated highly (r range 0.65 to 0.90, all P < 0.001).
Table 2.
Mean values of circulating cytokines in controls and ESRD patients
| Controls (N = 28) | Patients (N = 230) | ||||
|---|---|---|---|---|---|
| Mean | sd | Mean | sd | P | |
| IL-1, pg/ml | 9.7 | 3.1 | 87.8 | 112.0 | 0.0001 |
| IL-2, pg/ml | 11.6 | 3.3 | 15.8 | 7.7 | 0.008 |
| IL-4, pg/ml | 9.4 | 2.5 | 131.5 | 63.5 | 0.01 |
| IL-5, pg/ml | 8.5 | 2.4 | 127.4 | 60.9 | 0.002 |
| IL-6, pg/ml | 13.3 | 3.1 | 92.3 | 117.9 | 0.0001 |
| IL-12, pg/ml | 9.0 | 2.7 | 14.1 | 6.4 | 0.0002 |
| IL-13, pg/ml | 11.1 | 2.4 | 29.2 | 3.1 | 0.01 |
| TNF-α, pg/ml | 13.3 | 3.0 | 114 | 147 | 0.0001 |
| CH50, units/ml | 64.5 | 9.6 | 55.0 | 18 | 0.02 |
The mean immune parameters for the entire patient population are outlined in Table 2. Two distributional patterns were noted. In the first set (T-cell number and recall function, CH50, IL-2, IL-4, IL-5, and IL-12), relatively low levels of skewness were noted (Fig. 1). In the second case (IL-1, IL-6, IL-13, and TNF-α) the distribution was highly positively skewed (Fig. 2). In all cases, circulating cytokine levels were higher in patients treated with HD compared with the normal controls (Table 2).
Fig. 1. Distribution of immune parameters in 230 stable outpatients with end-stage renal disease (ESRD) treated with hemodialysis.
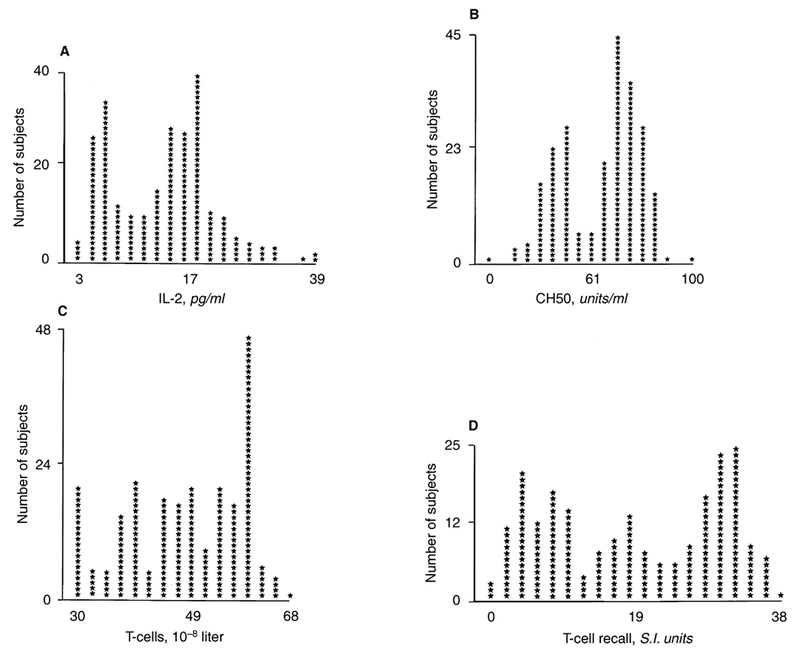
Distributions of IL-2 (A), CH50 (B), T-cell number (C) and T-cell function (D) show moderate skew (skewness values ranging from −0.45 to 0.33). These factors were associated with improved patient survival.
Fig. 2. Distribution of immune parameters in 230 stable outpatients with ESRD treated with hemodialysis.
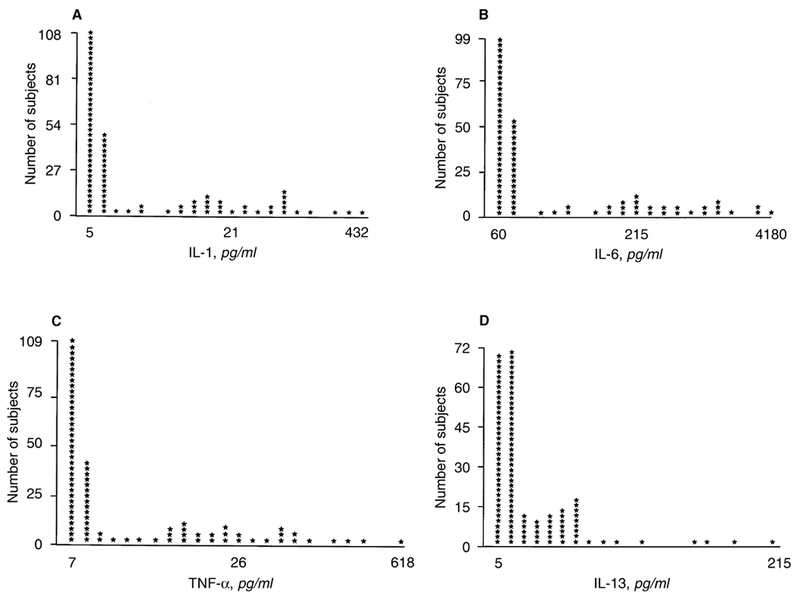
Highly positively skewed distributions of circulating IL-1 (A), IL-6 (B), TNF-α (C) and IL-13 (D) levels were noted in this population (skewness values ranging from 1.25 to 3.3). Most patients’ values were within a lower range, with a minority having extremely elevated values. These factors were associated with increased patient mortality.
Correlations of medical, nutritional, dialytic and immunologic data
Patients’ serum albumin concentrations were correlated with levels of Kt/V (r = 0.18, P = 0.008) and PCR (r = 0.27, P = 0.0001), and their circulating and log transformed IL-13 and TNF-α levels (r = 0.17, P = 0.01, r = 0.19, P = 0.005; r = 0.17, P = 0.005, r = 0.17, P = 0.01, respectively). In contrast, higher serum albumin concentrations were correlated with lower circulating and log transformed IL-2 levels (r = −0.17, P = 0.009, and r = −0.18, P = 0.008, respectively), T-cell number (r = −0.23, P = 0.0006) and T-cell antigen recall function (r = −0.19, P = 0.004). Lower T-cell antigen recall function correlated with higher values of PCR (r = −0.21, P = 0.002), but there was no correlation of T-cell number or function with Kt/V. There was no correlation of any other immune parameter and medical or demographic factor, including anthropometric indices. There was no correlation of the duration of the patients treatment for ESRD with any circulating cytokine. In fact, the correlation that most closely approached the level of significance (r = −0.14, P = 0.03) showed that IL-5 levels were higher in patients more recently starting ESRD therapy. Levels of all untransformed and log-transformed immune parameters, however, were all highly intercorrelated (P = 0.001).
Survival analysis
Because of the paucity of differences between mean levels of immunologic parameters between groups of patients treated with different types of dialyzers, the small number of patients treated with modified cellulose dialyzers, and the findings of Hakim et al [7], survival analyses were performed comparing patients treated with unmodified cellulose dialyzers with the group treated with modified cellulose and synthetic dialyzers (Table 3). There was no difference in mean age, severity coefficient, serum albumin concentration, or percentage of patients with diabetes mellitus between patients treated with unmodified cellulose dialyzers and other types of dialyzers (Table 3). Mean Kt/V was higher in the patients treated with modified and synthetic dialyzers, compared with those treated with unmodified cellulose dialyzers (1.29 ± 0.34 vs. 1.17 ± 0.27; P = 0.006, unpaired _t_-test). There was no difference in mean CH50 or cytokine (IL-1, IL-2, IL-4, IL-5, IL-6, IL-12, IL-13, or TNF-α) levels, T-cell number or T-cell recall function between the two groups.
Table 3.
Characteristics of patients treated with different dialyzers
| Modified cellulose and synthetic dialyzers | Unmodified cellulose dialyzers | |||||
|---|---|---|---|---|---|---|
| N | Mean | sd | N | Mean | sd | |
| Age, years | 126 | 54.2 | 13.7 | 104 | 54.6 | 15.0 |
| Diabetes, % | 52.6 | 47.4 | ||||
| Severity coefficient | 2.4 | 0.94 | 2.4 | 1.0 | ||
| Serum albumin, g/dl | 3.9 | 0.42 | 3.8 | 0.5 | ||
| Kt/V | 1.29 | 0.35 | 1.17 | 0.27a | ||
| IL-1, pg/ml | 82.3 | 104.4 | 94.7 | 120.8 | ||
| IL-2, pg/ml | 16.6 | 7.6 | 15.4 | 7.9 | ||
| IL-4, pg/ml | 12.5 | 6.0 | 13.9 | 6.7 | ||
| IL-5, pg/ml | 12.3 | 5.9 | 13.3 | 6.3 | ||
| IL-6, pg/ml | 89.8 | 115.2 | 95.4 | 121.7 | ||
| IL-12, pg/ml | 14.3 | 6.5 | 14.0 | 6.4 | ||
| IL-13, pg/ml | 30.4 | 35.0 | 27.9 | 25.2 | ||
| TNF-α, pg/ml | 106.6 | 140.7 | 123.9 | 154.5 | ||
| T-cells/10−8/liter | 49.5 | 10.1 | 50.2 | 9.8 | ||
| T-cell RECALL, SI units | 18.5 | 11.0 | 20.2 | 11.4 | ||
| CH50, units/ml | 54.1 | 0.3 | 55.4 | 18.2 |
A Cox regression applied to the entire sample confirmed an increased mortality risk for each decade increase in age (50%, P < 0.0001), for patients with compared to those without diabetes mellitus (57%, P < 0.05), and for a one unit increase in severity coefficient (80%, P = 0.0001; Table 4). There was a 30% decrease in risk for each 1 g/dl increase in serum albumin concentration, controlling for severity coefficient (P = 0.007). An association between increased mortality risk and gender or level of Kt/V, controlling for severity coefficient, could not be demonstrated in this comparatively small population. Although patients treated with modified cellulose and synthetic dialyzers had a 34% decreased mortality risk compared with those treated with unmodified cellulose dialyzers, controlling for severity coefficient, the difference did not quite achieve the level of significance (P = 0.08).
Table 4.
Medical relative mortality risk factors in 230 hemodialysis patients
| Risk factor | Alive | Died | Range | RR and 95% CI | P value | |
|---|---|---|---|---|---|---|
| 1 | Age (in decades) | 153 | 77 | 1.9–8.4 | 1.5 (1.3, 1.8) | < 0.0001 |
| 2 | Diabetes | 153 | 77 | 1.0–2.5 | 1.57 (1.4, 1.8) | < 0.05 |
| 3 | Severity | 153 | 77 | 1.4–2.2 | 1.8 (1.7, 1.9) | < 0.0001 |
| 4 | Albumin | 153 | 77 | 0.3–0.8 | 0.7 (0.6, 0.9) | 0.007 |
| 5 | Dialyzer Type | 153 | 77 | 0.4–1.1 | 0.66 (0.4, 1.04) | 0.08 |
Doubling of levels of circulating proinflammatory cytokines IL-1, and TNF-α were associated with a 72% and 65% increase in mortality risk in this patient population (C.I. 1.37 to 2.15 and 1.31 to 2.08 respectively, P < 0.0001), controlling for variations in age, severity coefficient, serum albumin concentration, dialyzer type and study site (Table 5). In contrast, higher levels of circulating IL-2, T-cell number and function, and CH50 were associated with improved survival (35%, 38%, 35% and 35% decrease in mortality risk, P < 0.001, < 0.0001, < 0.001, and < 0.001, respectively). Doubling of levels of circulating IL-4, IL-5 and IL-12 were associated with improved survival as well (36%, 34% and 24% decrease in mortality risk, P < 0.001, < 0.001 and = 0.02, respectively), while 100% increases in levels of IL-6, and IL-13 were associated with a 56%, and 58% increase in mortality risk in this patient population (P < 0.0001 and < 0.001, respectively). There was no difference in these findings when the association of the immune factors was controlled solely for age, severity of comorbid illness and level of serum albumin concentration, with the exception that the relative risk of the association of survival with the level of circulating IL-12 decreased to 0.82 (C.I. 0.65 to 1.03, P = 0.09). Similarly, there was no difference in the results (compared with those outlined in Table 5) when only the patients who had been dialyzed for more than six months were assessed.
Table 5.
Immune parameters predicting mortality controlling for age, severity, dialyzer type, albumin and site
| Parameter | Standardized RR and 95% CI | P value |
|---|---|---|
| Log IL-1 | 1.72 (1.37,2.15) | < 0.0001 |
| Log IL-2 | 0.65 (0.51,0.81) | < 0.001 |
| Log IL-4 | 0.64 (0.51,0.80) | < 0.001 |
| Log IL-5 | 0.66 (0.52,0.83) | < 0.001 |
| Log IL-6 | 1.56 (1.25,1.94) | < 0.0001 |
| Log IL-12 | 0.76 (0.60,0.96) | 0.02 |
| Log IL-13 | 1.58 (1.25,2.00) | < 0.001 |
| Log TNF-α | 1.65 (1.31,2.08) | < 0.0001 |
| T-cell (div by 4) | 0.62 (0.49,0.78) | < 0.0001 |
| T-cell recall (div by 5) | 0.65 (0.51,0.83) | < 0.001 |
| CH50 (div by 60) | 0.65 (0.52,0.82) | < 0.001 |
DISCUSSION
Henderson, Koch, Dinarello and Shaldon proposed the “Interleukin Hypothesis” in 1983, suggesting that cytokine stimulation by dialysis membranes might be associated with many of the symptoms of uremia that persist, even in seemingly well dialyzed patients [25]. We have demonstrated that variations in levels of circulating cytokines in hemodialysis patients are associated with changes in mortality risk, in a patient population exhibiting demographic and mortality markers comparable to those in the United States ESRD program [1, 26, 27]. Proinflammatory cytokines such as IL-1 and TNF-α [28] were independently associated with mortality in HD patients, while circulating levels of IL-2 and IL-12, cytokines critical for T-cell growth and function [28], IL-4 and IL-5, T-cell number and functional status, and CH50 were independently associated with survival.
The pattern of cytokines elaborated, reflecting the level of activation and the interaction of the immune network in a given patient, may be more important than their individual levels. Indeed, our data suggest that levels of particular cytokines, while predictive of mortality, may not be tightly causally linked to effectors of outcome. For example, IL-1 (a mortality factor in this study) increases synthesis of IL-2 (a correlate of survival) by lymphocytes, suggesting its association with mortality must be mediated through other pathways. Similarly, IL-4 (associated with survival in this study) and IL-6 (associated with mortality) both decrease production of IL-1 and TNF-α by monocytic cells, suggesting more complex effector routes must be present to foster such outcomes in HD patients. Cytokines are thought to exert their major physiologic and pathophysiologic effects at local, cellular levels. The physiologic importance of circulating cytokines is unknown, but infusion of cytokines has been used in various in vivo animal models of disease pathogenesis. We have argued in the past that the best way to assess functional aspects of cytokine dysregulation is in an in vitro cell system, under controlled, stimulated conditions, normalizing for immune cell number and subset type, and the ionic and protein microenvironment [9, 29]. However, in light of recent technical advances, it is clearly easier to assess circulating cytokines in the clinical setting. The level of circulating cytokines may be affected by many parameters such as the level of residual renal function and dialyzer clearances, differential absorption of cytokines to different dialyzer materials, variable ultrafiltration rates, differential cellular production, and different renal and comorbid diseases in a population using heterogeneous drugs [9, 10]. Certain patterns of cytokines may, however, be critically associated with other common antecedent medical conditions or host factors that diminish survival in ESRD patients treated with dialysis, such as atopy [30], rendering them useful as markers. In this regard, circulating levels of IL-6 and TNF-α have been shown to correlate with disease severity in several illnesses [10, 31]. Additionally, increased levels of TNF-α and IL-6, and abnormal regulation of IL-10 have been associated with an abnormal outcome, diminished response to hepatitis B-virus vaccination, in patients with renal disease [32].
In this study, however, such immune factors were generally not associated with the measured medical, nutritional or dialytic parameters. In this study, we could not show a relationship between the level of circulating cytokines and the duration of the patients treatment for ESRD, suggesting the abnormally high levels were not solely associated with the extent of residual renal function. In fact, there was no difference in the interpretation of the results when only patients dialyzed for more than six months before study entry (presumed to have little residual renal function) were asssessed, suggesting the inclusion of incident patients with appreciable renal function had little impact on the prediction of survival. Recent reviews [29, 33] have emphasized the potential relationships between IL-1 and TNF-α and catabolism, muscle proteolysis and malnutrition, but these relationships could not be confirmed with anthropometric indices and nutritional parameters in this study. Paradoxically, higher serum albumin levels, often linked with survival in other studies of ESRD patients [reviewed in 10, 26], were associated with cytokines strongly predictive of mortality, such as IL-13, while lower albumin levels were associated with “protective” immune factors, such as IL-2 and T-cell number and function, corroborating the nutrition-independent associations of the immune parameters with survival in this study. This may be due to the role of some cytokines as acute phase reactants, which are associated with diminution in serum albumin levels [10, 26, 28]. Alternatively, the circulating cytokine levels may not be closely associated with certain tightly-regulated cellular physiological processes. The interaction of these systems may ultimately be as important to patient survival as the provision of optimal dialysis. While immune imbalance may be associated with mortality, since individual cytokines have multiple synergistic and antagonistic actions and affect different cellular targets, particular cytokine combinations may have unpredictable effects on various cellular and organ systems, leading to differential outcomes.
Additionally, the source of the circulating cytokines measured in the study, and their mechanistic role in affecting outcome are unclear. TNF-α, IL-1 and IL-6 are made by many cells, while IL-2, IL-4 and IL-5 are only synthesized by lymphoid cells [28]. IL-4 is critical in regulating B-lymphocyte function and circulating immunoglobulin levels, and blocks monocyte production of IL-1, IL-6 and TNF-α. Both IL-2 and IL-4 increase T-cell maturation and cytotoxicity [34]. One interpretation of the current findings is that cytokines reflecting monocytic cell activation are associated with poor outcome, while better T cell function and humoral immunity are associated with a survival advantage in patients treated with hemodialysis. This interpretation is bolstered by the findings related to outcome documented by Girndt et al [32]. These effects may be mediated through resistance to infection, the second most prevalent cause of death in the ESRD population [1]. Such notions are also supported by our finding of the association of increased levels of CH50, a measure of the ability to opsonize and lyse bacteria, clear immune complexes, and enhance neutrophil, monocyte and B-lymphocyte function, with enhanced survival [35]. Another possibility is that patients with the highest levels of proinflammatory cytokines have reacted to the presence of endotoxins, secondary to infection or as a complication of dialysis [36]. The presented data cannot distinguish between these possibilities.
Recent studies demonstrated a survival advantage in patients treated with modified cellulose or synthetic dialyzers [7]. The authors considered that differences in their “biocompatibility” resulting in less immune stimulation in patients treated with synthetic or modified dialyzers might underlie this effect, or that other factors, such as differences in gross amount or type of molecular clearance, dialytic bath or mechanical factors might be associated with improved survival. With the exception of T-cell recall function, there were no differences in immune parameters measured before the start of a dialysis session between groups of patients treated with the various types of dialyzers in this study. Our findings suggest that differences in patients’ pre-treatment immune function are quantitatively relatively more important than the type of dialyzer used in their treatment, but do not directly underlie differential survival in patients treated with different types of dialyzers. Rather, the higher dose of dialysis, or differences in middle molecule clearance may be related to survival in patients treated with newer types of dialyzers. We expect that with a larger number of patients who are followed for a longer period of time, however, the differences in survival between patients treated with different types of dialyzers would reach the level of statistical significance. Whether differential effects on patients’ immune status after hemodialysis are associated with these findings must be addressed in further studies.
Variations in the immune parameters were associated with changes in the relative risk of mortality in the range of magnitude of effects that age, presence of diabetes mellitus, severity of comorbid illness and level of serum albumin concentration displayed, even when the variations of these parameters were controlled in the analysis. We could not demonstrate an association between immune parameters and Kt/V or PCR. Our population had a relatively high Kt/V and adequate nutritional status [15, 16, 27]. The effect of increasing the dose of dialysis provided may be seen only in relatively large populations with longer observation periods. Additionally, there may be diminished returns of increasing delivery of dialysis when the Kt/V achieved is already high. Such questions are currently being addressed in prospective studies.
Our study population was primarily comprised of African-American, urban patients. Our present findings must be replicated in larger and more diverse populations to establish the generalizable and specific findings reported. In addition, because of the exploratory nature of the present study, we did not measure the level of circulating cytokines after hemodialysis. We and others [9; reviewed in 10, 30, 32, 37] have previously shown that cytokine metabolism may alter markedly after treatment. The relationship between immune parameters before and after dialysis, and the effect of interventions designed to normalize the immunologic status of ESRD patients treated with hemodialysis on survival should be studied.
ACKNOWLEDGMENTS
This work was supported by NIH grants 1-RO1-DK 45578 (P.L.K.), and ZO1-HD-00641-01 (J.A.Y.) and the ORMH (J.A.Y.), National Institutes of Health.
We are grateful to Juan P. Bosch, M.D., Michael A. Kolber, Ph.D., M.D., Gary Mishkin, M.S., Joseph A. Vassalotti M.D., and Prudence P. Kline, M.D. for preliminary discussions regarding these findings. We thank the nursing, social work and dietary staff of the outpatient hemodialysis units of the GAMBRO-George Washington University Medical Center, the Howard University Medical Center, and the Washington Veterans Affairs Medical Center, for cooperating in this study. We are indebted to the hard working research staff and volunteers (Nicole Shidler, Julie Kovac, Deneane Boyle, Maria Whittington, Deatrice Williams, Ilse Wendorf, Kaidi Fullerton, Kirti Sharma, Ghada Swadek, Mark Collier, and Beth Lorell), who collected and entered these data. We also thank Ms. Nancy Armistead and the staff of ESRD Network 5, who provided data on vital statistics of patients enrolled in the study. Most of all, we thank our patients who continue to teach us about chronic renal disease, for volunteering to participate in this study. These findings were presented, in part, at the 14th Congress of the International Society of Nephrology, Sydney, Australia, May 1997 and the 30th Annual Meeting of the American Society of Nephrology, San Antonio, Texas, USA, November 1997.
REFERENCES
- 1.Held PJ, Port FK, Webb RL, et al. : Excerpts from the United States Renal Data System 1996 Annual Report. Am JKidney Dis 28(Suppl): S79–S92, 1996 [Google Scholar]
- 2.Owen WF Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM: The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 329: 1001–1006, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Lowrie EG, Lew NL: Death risk in hemodialysis patients: The predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis 15:458–482, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Lowrie EG, Laird M, Parker TF, Sargent JA: The effect of hemodialysis prescription on patient mortality. Report from the NCDS. N Engl J Med 32:382–389, 1981 [DOI] [PubMed] [Google Scholar]
- 5.Held PJ, Port FK, Wolfe RA, Stannard DC, Carroll CE, Daugirdas JT, Bloembergen WE, Greer JW, Hakim RM: The dose of hemodialysis and patient mortality. Kidney Int 50:550–556, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Hakim RM, Breyer J, Ismail N, Schulman G: Effects of dose of dialysis on morbidity and mortality. Am J Kidney Dis 23:661–669,1994 [DOI] [PubMed] [Google Scholar]
- 7.Hakim RM, Held PJ, Stannard DC, Wolfe RA, Port FK, Daugirdas JT, Agodoa L: Effect of the dialysis membrane on mortality of chronic hemodialysis patients. Kidney Int 50:566–570, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Raska K, Raskova J, Shea SM, Frankel RM, Wood RH, Lifter J, Ghobrial I, Eisinger RP, Homer L: T cell subsets and cellular immunity in end-stage renal disease. Am J Med 75:734–740, 1983 [DOI] [PubMed] [Google Scholar]
- 9.Kimmel PL, Phillips TM, Phillips E, Bosch JP: The effect of renal replacement therapy of cellular cytokine production in patients with renal disease. Kidney Int 38:129–135, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA: Cytokines: Agents provacateurs in hemodialysis? Kidney Int 41:683–694, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Kay NE, Raij LR: Immune abnormalities in renal failure and hemodialysis. Blood Purif 4:120–129, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR: Mini-mental state: A practical method for grading the cognitive states of patients for the clinician. J Psych Res 12:189–198, 1975 [DOI] [PubMed] [Google Scholar]
- 13.Plough AL, Shwartz M, Salem SR, Weller JM, Ferguson CW: Severity analysis in end stage renal disease: A risk group approach. Am Soc Artif Intern Org J 8:33–40, 1985 [Google Scholar]
- 14.Jindal JK, Manuel A, Goldstein MB: Percent reduction in blood urea concentration during hemodialysis. Trans Am Soc Artif Intern Organs 33:286–288, 1987 [PubMed] [Google Scholar]
- 15.Durnin JVGA, Womersley J: Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32:77–96, 1974 [DOI] [PubMed] [Google Scholar]
- 16.Heymsfield SB, McManus C, Smith J, Stevens V, Nixon DW: Anthropometric measurement of muscle mass: Revised equations for calculating bone-free arm muscle area. Am J Clin Nutr 36:680–690, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Kimmel PL, Peterson RA, Weihs KL, Simmens SJ, Boyle DH, Verme D, Umana WO, Veis JH, Alleyne S, Cruz I: Behavioral compliance with dialysis prescription in hemodialysis patients. J Am Soc Nephrol 5:1826–1834, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Schellekens P, Eijsvoogoz VP: Lymphocyte transformation in vitro. i. Tissue culture conditions and qualitative measurement. Clin Exp Immunol 3:571–578, 1968 [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips TM: Analytical Techniques in Immunochemistry. New York, Marcel Dekker, 1992, pp 195–196, 281–283 [Google Scholar]
- 20.Phillips TM: High performance capillary electrophoresis analysis of tissue cytokines. LC.GC 11:36–42, 1993 [Google Scholar]
- 21.Phillips TM, Kimmel PL: High performance capillary electrophoresis analysis inflammatory citokines in human biopsies. J Chromatog Biomed Appl 656:259–266, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Thorpe GhG, Bronstein I, Kricka LJ, Edwards B, Voyta JC: Chemiluminescent enzyme immunoassay of _α_-fetoprotein based on an adamentyl dioetane phenyl phosphate substrate. Clin Chem 35:2319–2321, 1989 [PubMed] [Google Scholar]
- 23.Lee EL: Statistical Methods for Survival Data Analysis (2nd ed). New York, Wiley, 1992, pp 250–263 [Google Scholar]
- 24.Hosmer D, Lemeshow S: Applied logistic regression, in Statistical Methods for Survival Data Analysis (2nd ed). New York, John Wiley & Sons, Inc., 1989, pp 56–57 [Google Scholar]
- 25.Henderson LW, Koch KM, Dinarello CA, Shaldon S: Hemodialysis hypotension: The interleukin hypothesis. Blood Purif 1:3–8, 1983 [Google Scholar]
- 26.Ikizler TA, Hakim RM: Nutrition in end-stage renal disease. Kidney Int 50:343–357, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Nelson EE, Changgi DH, Pesce AL, Peterson DW, Singh S, Pollack VE: Anthropometric norms for the dialysis population. Am J Kidney Dis 16:32–37, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Vilcek J, Le J: Immunology of cytokines: An introduction, in The Cytokine Handbook (2nd ed), edited by Thomson A, London, Academic Press Ltd, 1994, pp 1–19 [Google Scholar]
- 29.Kimmel PL, Phillips tM, Lew SQ, Langman CB: Zinc modulates mononuclear cell calcitriol metabolism in peritoneal dialysis patients. Kidney Int 49:1407–1412, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Hertel J, Kimmel PL, Phillips TM, Bosch JP: Eosinophilia and cellular cytokine responsiveness in hemodialysis patients. J Am Soc Nephrol 3:1244–1252, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Fisher CJ Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RMH, Benjamin E: Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. N Engl J Med 334:1697–1702, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Girndt M, Kohler H, Schiedheim-Weick E, Schlaak JF, zum Buschenfelde K- HM, Fleischer B: Production of interleukin-6. tumor necrosis factor-α and interleukin-10 in vitro correlates with the clinical immune defect in chronic hemodialysis patients. Kidney Int 47:559–565, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Mitch WE, Goldberg AL: Mechanisms of muscle wasting: The role of the ubiquitin-proteasome pathway. N Engl J Med 335:1897–1905, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Banchereau J, Ryback ME: Interleukin 4, in The Cytokine Handbook (2nd ed), edited by Thomson A, London, Academic Press Ltd, 1994, pp 99–126 [Google Scholar]
- 35.Weiler JM: Introduction, in Complement in Health and Disease (vol 20), Immunology and Medicine, edited by Whaley K,Loos M, Weiler JM, Hingham, Kluwer Academic Publishers, 1993, pp 1–37 [Google Scholar]
- 36.Pereira BJG, Snodgrass BR, Hogan PJ, King AJ: Diffusive and convective transfer of cytokine-inducing bacterial products across hemodialysis membranes. Kidney Int 47:603–610, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Pereira BJG, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA: Plasma levels of IL-1 beta, TNF-alpha and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int 45:890–896, 1994 [DOI] [PubMed] [Google Scholar]