Selective Activation of Gαo by D2LDopamine Receptors in NS20Y Neuroblastoma Cells (original) (raw)
Abstract
D2L dopamine receptor activation results in rapid inhibition and delayed heterologous sensitization of adenylate cyclase in several host cell types. The D2L dopamine receptor was stably transfected into NS20Y neuroblastoma cells to examine inhibition and sensitization in a neuronal cell environment and to identify the particular G-proteins involved. Acute activation of D2Lreceptors with the selective D2 agonist quinpirole inhibited forskolin-stimulated cAMP accumulation, whereas prolonged incubation (2 hr) with quinpirole resulted in heterologous sensitization (more than twofold) of forskolin-stimulated cAMP accumulation in NS20Y-D2L cells. To unambiguously identify the pertussis toxin (PTX)-sensitive G-proteins responsible for inhibition and sensitization, we used viral-mediated gene delivery to assess the ability of genetically engineered PTX-resistant G-proteins (Gαi1*, Gαi2*, Gαi3*, and Gαo*) to rescue both responses after PTX treatment. The expression and function of individual recombinant G-proteins was confirmed with Western blotting and inhibition of GTPγS-stimulated adenylate cyclase, respectively. To assess the specificity of D2L-Gα coupling, cells were infected with herpes simplex virus (HSV) recombinants expressing individual PTX-resistant G-protein α subunits and treated with PTX, and quinpirole-induced responses were measured. Infection of NS20Y-D2L cells with HSV-Gαo* rescued both inhibition and sensitization in PTX-treated cells, whereas infection with HSV-Gαi1*, HSV-Gαi2*, or HSV-Gαi3* failed to rescue either response. In summary, the current study provides strong evidence that the D2L dopamine receptor couples to Gαoin neuronal cells, and that this coupling is responsible for both the acute and subacute effects of D2 receptor activation on adenylate cyclase activity.
Keywords: dopamine D2L receptors, Gαi/o, NS20Y neuroblastoma, adenylate cyclase, pertussis toxin, heterologous sensitization
Alterations in D2-like dopamine receptors and their signaling pathways are thought to be involved in the etiology and treatment of many neuropsychiatric disorders, including schizophrenia, depression, Parkinson’s disease, and drug abuse. Hence, identifying the signaling pathways evoked after D2-like dopamine receptor activation may help us to understand the biochemical changes that occur in clinical settings. Such studies in native neuronal tissues are made difficult by the molecular diversity of D2-like dopamine receptors (D2S, D2L, D3, and D4), the number of pertussis toxin (PTX)-sensitive G-proteins through which they can couple (Gαi1, Gαi2, Gαi3, Gαoa, and Gαob), and the many signal pathways modulated by D2-like receptor activation (Huff, 1997; Watts and Neve, 1997).
One of the characteristic features of D2-like dopamine receptors and other Gαi/o-coupled receptors is PTX-sensitive inhibition of cAMP accumulation. Also, persistent stimulation of Gαi/o-coupled receptors such as the D2-like dopamine receptors and the μ opioid receptor results in the sensitization of adenylate cyclase to subsequent stimulation (Sharma et al., 1975; Bates et al., 1991; Ammer and Schulz, 1996; Watts and Neve, 1996; Watts et al., 1998). Heterologous sensitization has been proposed to be one mechanism by which a cell adapts to prolonged inhibition of cAMP synthesis and may be a cellular model of drug tolerance and dependence (Sharma et al., 1975; Ammer and Schulz, 1996; Nestler and Aghajanian, 1997). Although both D2-mediated inhibition and heterologous sensitization of forskolin-stimulated cAMP accumulation are blocked by PTX, no studies have directly examined and compared the G-protein specificity for these signaling events. Moreover, heterologous sensitization does not appear to be a direct result of decreased intracellular cAMP levels or reduced PKA activity (Watts and Neve, 1996; Watts et al., 1998), raising the possibility that different PTX-sensitive G-proteins mediate inhibition and heterologous sensitization of adenylate cyclase.
Among the approaches that have been used to identify the G-proteins that mediate D2 dopamine receptor signaling are antisense oligonucleotide treatments (Liu et al., 1994), the use of Gα subunit-specific antibodies (Lledo et al., 1992; Izenwasser and Côté, 1995), and rescue with PTX-insensitive Gα subunits (Senogles, 1994; O’Hara et al., 1996). In the latter approach, expression of individual PTX-resistant α subunits is used to determine the specificity of receptor-G-protein signaling after elimination of endogenous receptor-G-protein coupling by treatment with PTX (Taussig et al., 1992).
We used a defective herpes simplex virus vector (HSV) for acute expression of PTX-insensitive mutants to compare the D2L:Gα protein specificity for inhibition and heterologous sensitization of cAMP accumulation in a neuronal-like environment. The D2L dopamine receptor stably expressed in NS20Y cells (NS20Y-D2L) exhibited the appropriate pharmacological and functional properties of endogenous D2dopamine receptors. Viral-mediated gene delivery of recombinant Gα subunits provided rapid (18 hr) expression of functional proteins in NS20Y-D2L cells. We now report that stimulation of D2L receptors in NS20Y-D2L cells preferentially activates Gαo for both the inhibition and sensitization of forskolin-stimulated cAMP accumulation.
MATERIALS AND METHODS
Materials. [3H]cAMP was purchased from NEN Life Science Products (Boston, MA), and [3H]spiperone (104 Ci/mmol) was purchased from Amersham Life Sciences (Arlington Heights, IL). Quinpirole, (+)-butaclamol, and forskolin were purchased from Research Biochemicals International (Natick, MA). Dopamine (3-hydroxytyramine), pertussis toxin, growth media, and most other reagents were purchased from Sigma (St. Louis, MO). Fetal bovine serum (FBS) and iron-supplemented calf bovine serum (CBS) were purchased from HyClone (Logan, UT). Antisera to Gαi1 and Gαi2 were purchased from Signal Transduction (San Diego, CA). Antisera to Gαi3 and Gαo were generously provided by Dr. David Manning (University of Pennsylvania, Philadelphia, PA). cDNAs encoding the pertussis toxin-resistant G-proteins were generously provided by Dr. Ronald Taussig (University of Michigan, Ann Arbor, MI).
Production and maintenance of cell lines. Transfection of NS20Y cells with the D2L vector was performed by calcium phosphate precipitation as described previously (Cox et al., 1992). The plasmids pcDNA1-D2L (20 μg) and pBabe Puro (2 μg) were mixed with 0.5 ml of 0.25 m CaCl2, and 0.5 ml of 2× BBS [50 mm_N,N_-bis-(2-hydroxyethyl)-2-amino-ethanesulfonic acid, 280 mm NaCl, 1.5 mm NaHPO4] was added. The mixture was incubated for 25 min and added dropwise to exponentially growing NS20Y cells in a 10 cm tissue culture plate. Transfectants were isolated and screened by [3H]spiperone binding as described previously (Cox et al., 1995). NS20Y-D2L cells were maintained in DMEM supplemented with 5% FBS and 5% CBS, penicillin–streptomycin, and puromycin (2 μg/ml). Cells were grown in a humidified incubator at 37°C in the presence of 10% CO2.
Generation and packaging of HSV vectors. The construction of PTX-resistant mutant Gα (Gα*) cDNAs in which a serine replaced a cysteine four residues from the C terminus has been described previously (Taussig et al., 1992; O’Hara et al., 1996). Mutant cDNAs were cloned into pHSVPrPUC using standard molecular techniques, and replication-defective HSV vectors expressing mutant Gα subunits were prepared as described (Neve et al., 1997). The titer of the helper virus component of each stock was 1–1.2 × 103plaque-forming units/μl on 2–2 cells. HSV-LacZ was prepared simultaneously with the individual HSV-Gα subunit vectors with a titer of 2 × 105 infectious units/μl. Expression of the HSV-Gα* subunit vectors was confirmed by Western blotting (see Results).
Radioligand binding assays. Confluent cells in 10 cm plates were harvested by lysis with ice-cold hypotonic buffer (1 mm Na+-HEPES, pH 7.4, 2 mmEDTA). After swelling for 10–15 min, the cells were scraped from the plate and spun at 24,000 × g for 20 min. The resulting crude membrane fraction was resuspended in Tris-buffered saline (50 mm Tris-HCl, pH 7.4, with 155 mm NaCl) with a Brinkmann Polytron homogenizer (Westbury, NY) at setting 6 for 10 sec and used for radioligand binding assays. The binding of [3H]spiperone was assessed as described previously (Starr et al., 1995; Watts and Neve, 1996). Aliquots of the membrane preparation (5–15 μg of protein) were added to duplicate assay tubes containing the following: Tris-buffered saline, 0.001% bovine serum albumin, radioligand, and appropriate drugs. (+)-Butaclamol (2 μm) was used to define nonspecific binding. Incubations were performed at 37°C for 45 min, in a volume of 1.0 ml, and terminated by filtration using a 96-well Tomtec cell harvester (Orange, CT). Filters were allowed to dry, and 50 μl of BetaPlate scintillation fluid was added to each sample. Radioactivity on the filters was determined using a BetaPlate scintillation counter (LKB-Wallac, Gaithersburg, MD).
cAMP accumulation assays. Cells were seeded at a density of 250,000–300,000 cells/well in 24-well tissue culture clusters. Experiments used confluent cells and were completed in assay buffer (Earle’s balanced salt solution, containing 15 mm HEPES, 1 mm isobutylmethylxanthine, 2% CBS, and 0.02% ascorbic acid). For inhibition experiments, cells were preincubated with 300 μl of assay buffer for 10 min and placed on ice. D2agonists in the absence or presence of antagonists were added to wells before the addition of 10 μm forskolin. For sensitization experiments, cells were preincubated for 2 hr in the presence of drugs at 37°C in a humidified incubator with 10% CO2 and then washed three times for 3–4 min each with 300 μl of assay buffer. Forskolin (10 μm) was then added in the presence of spiperone (1 μm) to preclude acute effects of D2 dopamine receptor activation by residual agonist (Watts and Neve, 1996). cAMP accumulation for inhibition and sensitization experiments was performed for 15 min at 37°C, the assay buffer was decanted, and the cells were placed on ice and lysed with 3% trichloroacetic acid. The 24-well plates were then centrifuged at 1000 × g for 15 min and stored at 4°C for at least 1 hr before quantification of cAMP. For functional studies using HSV recombinants, confluent NS20Y-D2L cells were infected with viral preparations (approximately one infectious unit/cell) in 1 ml of growth medium for 18 hr, the medium was removed and replaced with medium containing PTX, as indicated in the figure legends, and then sensitization or inhibition experiments were completed as described above.
Adenylate cyclase assay in NS20Y-D2L cell membranes. Confluent cell monolayers in six-well tissue culture plates were infected with viral preparations (approximately one infectious unit/cell) for 18 hr in fresh growth medium. Cells were harvested by lysis with ice-cold hypotonic buffer (2 mmNa+-HEPES, pH 7.4, 2 mm EDTA, 1 mm DTT, and 0.3 mm PMSF), and the cell lysates were scraped from the plate, homogenized with a Brinkmann Polytron homogenizer at setting 6 for 5 sec, and spun at 30,000 ×g for 20 min. The resulting crude membrane fraction was resuspended (∼1 mg/ml) in storage buffer (15 mmNa+-HEPES, pH 7.4, 2 mm EDTA, 1 mm DTT, and 0.3 mm PMSF) and frozen at −70°C until assayed. Adenylate cyclase assays were performed as described previously with modifications (Watts et al., 1995a). Frozen membranes were thawed and added (10–20 μg of protein/tube) to duplicate assay tubes containing the reaction mixture (15 mmNa+-HEPES, pH 7.4, 20 mmphosphocreatine, 1 mm MgCl2, 2 mm ATP, 1 mm isobutylmethylxanthine, 5 U of creatine phosphokinase) and forskolin (30 μm) in the absence or presence of GTPγS (1 μm), in a final volume of 100 μl. Incubations were performed for 15 min at 30°C and terminated by the addition of 3% trichloroacetic acid. Tubes were vortexed and centrifuged for 10 min at 15,000 × g. cAMP in the supernatant was quantified as described below.
Quantification of cAMP. cAMP was quantified using a competitive binding assay adapted with minor modifications fromNordstedt and Fredholm (1990). Duplicate samples of the cell lysate (5–10 μl) were added to reaction tubes containing cAMP assay buffer (100 mm Tris-HCl, pH 7.4, 100 mm NaCl, 5 mm EDTA). [3H]cAMP (1 nmfinal concentration) was added to each assay, followed by cAMP-binding protein (∼100 μg of crude adrenal extract in 500 μl of cAMP buffer). The reaction tubes were incubated on ice for 2 hr and harvested by filtration as described for radioligand binding assays. cAMP concentrations in each sample were estimated in duplicate from a standard curve ranging from 0.1 to 100 pmol cAMP/assay.
Membrane preparation for immunodetection. For the standard membrane preparation, cells were infected with viruses and harvested as described for membrane adenylate cyclase assays. The resulting membrane pellet was resuspended in 80 μl of HEPES buffer (15 mmNa+-HEPES, pH 7.5, 1.0 mm DTT, and 0.3 mm PMSF), and protein concentration was determined using a BCA protein assay (Pierce, Rockford, IL). Proteins were equalized by dilution in SDS-PAGE sample buffer and frozen at −70°C until use. For the enriched preparation, membranes were prepared as described previously (Watts et al., 1998). Cells were scraped with a rubber policeman in HEPES buffer with 0.25 m sucrose. The cells were collected by centrifugation at 1,000 × g for 10 min, homogenized with a Teflon-glass homogenizer (eight strokes), and then centrifuged at 600 × g to remove the nuclei. The supernatant was decanted, and the resulting nuclear pellet was resuspended (two to three strokes) and centrifuged at 1000 ×g for 10 min. The supernatants were pooled and centrifuged at 48,000 × g for 10 min. The membrane pellet was resuspended in HEPES buffer and centrifuged at 48,000 ×g for 10 min. The final membrane pellet was resuspended in 400 μl of HEPES buffer and frozen at −70°C until use. Protein was determined as described above for the standard membrane preparation.
Immunodetection. Protein was subjected to SDS-PAGE through a 10% polyacrylamide gel and transferred to polyvinylidene difluoride (PVDF) membranes (Costar Corning, Cambridge, MA). Membrane sheets were blocked overnight with 5% nonfat dry milk, washed with Tris-buffered saline, and incubated with specific G-protein α subunit antibodies for 3 hr. The PVDF membranes were washed, and immunodetection was accomplished using the ECF Western blotting kit (Amersham Life Sciences, Buckinghamshire, England) according to the manufacturer’s instructions. Membranes were incubated with secondary antibody (fluoroscein-linked anti-rabbit Ig or fluoroscein-linked anti-mouse Ig) for 1 hr, washed, and then incubated with tertiary antibody (anti-fluoroscein-alkaline phosphatase conjugate) for 1 hr. Membranes were again washed, exposed to ECF substrate for 7 min, dried at room temperature for 20 min, and then scanned using a Storm Imaging System (Molecular Dynamics, Sunnyvale, CA).
Data analysis. Saturation isotherms for the binding of [3H]spiperone, competition binding studies, and dose–response curves for stimulation and inhibition of cAMP accumulation were analyzed by nonlinear regression using the program GraphPAD Prism (San Diego, CA). Statistical comparisons were made using ANOVA followed by Dunnett’s post hoc t test comparing vehicle or control with treated groups, except where indicated.
RESULTS
Pharmacological characterization of D2L dopamine receptors in NS20Y neuroblastoma cells
The density of binding sites in membranes prepared from NS20Y cells expressing the D2L receptor (NS20Y-D2L), determined by saturation analysis of [3H]spiperone binding, was 690 ± 50 fmol/mg of protein (n = 7) with a _K_D for [3H]spiperone of 68 ± 7 pm. Competition binding studies completed in the absence of GTP revealed shallow Hill slopes (_n_H) for both dopamine (0.57 ± 0.03, n = 5) and quinpirole (0.72 ± 0.02, n = 5) when competing for [3H]spiperone-labeled sites, consistent with an agonist profile. The Hill slope for the binding of sulpiride was 0.97 ± 0.07 (n = 3), consistent with an antagonist profile. The apparent affinity constants for each drug were 6.9 ± 0.7 μm (dopamine), 6.7 ± 0.8 μm (quinpirole), and 64 ± 2 nm(sulpiride).
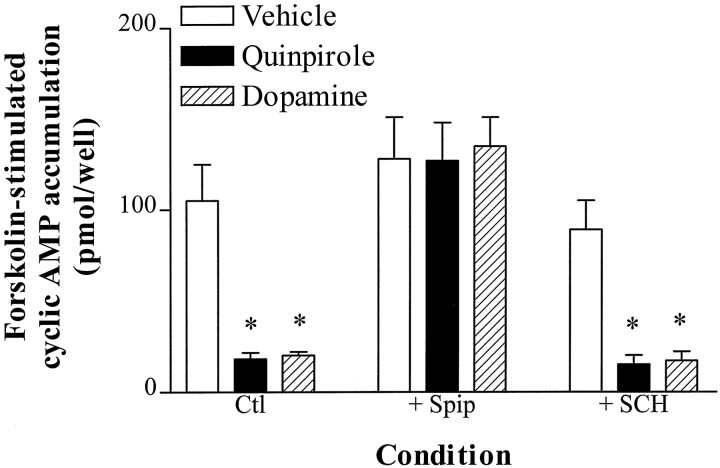
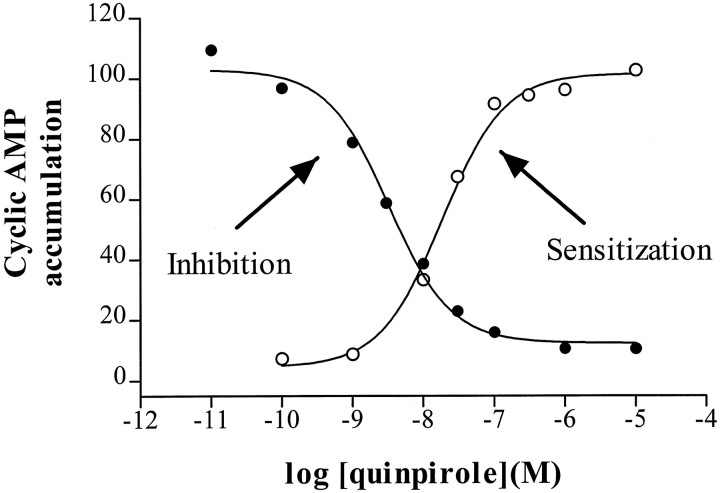
We examined the ability of D2 agonists to inhibit forskolin-stimulated cAMP accumulation in NS20Y-D2L cells. Dopamine and quinpirole markedly inhibited forskolin-stimulated cAMP accumulation, and the inhibition by both agonists was completely prevented by the D2 antagonist spiperone (Fig.1). Although NS20Y cells endogenously express low levels of D1-like dopamine receptors (Monsma et al., 1989), the selective D1 antagonist SCH23390 did not alter quinpirole- or dopamine-induced inhibition of cAMP accumulation in NS20Y-D2L cells (Fig. 1). Dose–response curves for inhibition of cAMP accumulation by quinpirole revealed an IC50 value of 4.1 ± 1.4 nm and a maximal inhibition of 88 ± 5% (Fig.2).
Fig. 1.
Specificity of D2L receptor-mediated inhibition of cAMP accumulation. cAMP accumulation was stimulated by forskolin (10 μm) in NS20Y-D2L cells in the absence or presence of quinpirole (1 μm) or dopamine (1 μm) for 15 min. As indicated in the figure, some experiments were performed in the presence of 1 μmspiperone (+ Spip) or 1 μm SCH23390 (+ SCH). Data shown are the mean ± SE of three to four independent experiments, each assayed in duplicate. *p < 0.01 compared with vehicle-treated cells (Dunnett’s post-repeated measures ANOVA).
Fig. 2.
Potency of quinpirole for inhibition and sensitization of forskolin-stimulated cAMP accumulation. Each point is the average of duplicate determinations, expressed as a percentage of forskolin-stimulated activity in the absence of quinpirole (•,Inhibition) or after 2 hr pretreatment with 10 μm quinpirole (○, Sensitization). Dose–response curves for quinpirole inhibition of cAMP accumulation were determined in NS20Y-D2L cells stimulated with 10 μm forskolin and increasing concentrations of quinpirole for 15 min. For sensitization, NS20Y-D2L cells were treated with increasing concentrations of quinpirole for 2 hr and washed, and cAMP accumulation was stimulated with 10 μm forskolin. The experiments shown are representative of three independent experiments. In the inhibition curve shown, the maximal inhibition was 88% and the IC50 value was 3.3 nm. Forskolin stimulation in the absence of quinpirole was 135 pmol/well. The EC50 value for sensitization was 18 nm; forskolin-stimulated cAMP accumulation was 130 pmol/well in vehicle-treated cells and 320 pmol/well in cells treated with 10 μm quinpirole.
Sensitization of forskolin-stimulated cAMP accumulation in NS20Y-D2L cells
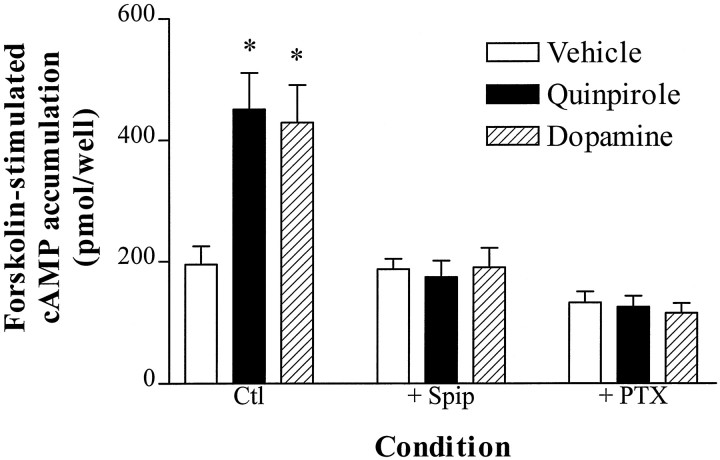
Although acute activation of D2L receptors in NS20Y cells inhibits forskolin-stimulated cAMP accumulation (Fig. 1), 2 hr treatment of NS20Y-D2L cells with dopamine or quinpirole enhanced subsequent forskolin-stimulated cAMP accumulation (Fig.3). This D2 agonist-induced heterologous sensitization was prevented by coincubation with spiperone or pretreatment with PTX (Fig. 3) but was not blocked by coincubation with the D1 antagonist SCH23390 (data not shown). The EC50 value for quinpirole-induced sensitization of forskolin-stimulated cAMP accumulation in NS20Y-D2L cells was 26 ± 4 nm, with a maximal increase in cAMP accumulation that was 134 ± 4% (n = 3) greater than vehicle-treated cells (Fig. 2).
Fig. 3.
D2 agonist-induced heterologous sensitization. NS20Y-D2L cells were treated with vehicle, quinpirole (1 μm), or dopamine (1 μm) for 2 hr at 37°C. Where indicated, some agonist treatments were completed in the presence of 1 μm spiperone (+ Spip) or after overnight treatment with 50 ng/ml pertussis toxin (+ PTX). Cells were extensively washed, and cAMP accumulation was stimulated with forskolin (10 μm) for 15 min. Data shown are the mean ± SE of four to six independent experiments, each assayed in duplicate. *p < 0.01 compared with vehicle-treated cells (Dunnett’s post-repeated measures ANOVA).
Expression and function of PTX-resistant G-protein α subunits (Gαi1*, Gαi2*, Gαi3*, and Gαo*) using the HSV vector
Before investigating the coupling of the D2L receptor to recombinant PTX-resistant Gα subunits (Gα*), we characterized the expression and function of Gα* subunits under the conditions to be used for D2L dopamine receptor functional assays. NS20Y-D2L cells were infected with HSV recombinants expressing individual Gα* subunits (HSV-Gα*), and expression was examined by Western blotting of membrane homogenates. Infection for 18 hr produced robust expression of each of the recombinant Gα* subunits (Fig. 4). We also examined the function and confirmed the PTX resistance of each Gα* subunit. After infection with HSV-Gα* viruses, cells were treated with PTX, and the effect of each HSV-Gα* subunit on GTPγS-stimulated cAMP accumulation was measured. In control and HSV-LacZ-infected cells, the addition of GTPγS (1 μm) significantly potentiated forskolin-stimulated cAMP accumulation by ∼100% (Table1). In contrast, infection with the inhibitory Gα subunits HSV-Gαi1*, -Gαi2*, -Gαi3*, or -Gαo* prevented GTPγS potentiation of forskolin-stimulated cAMP accumulation (Table 1).
Fig. 4.
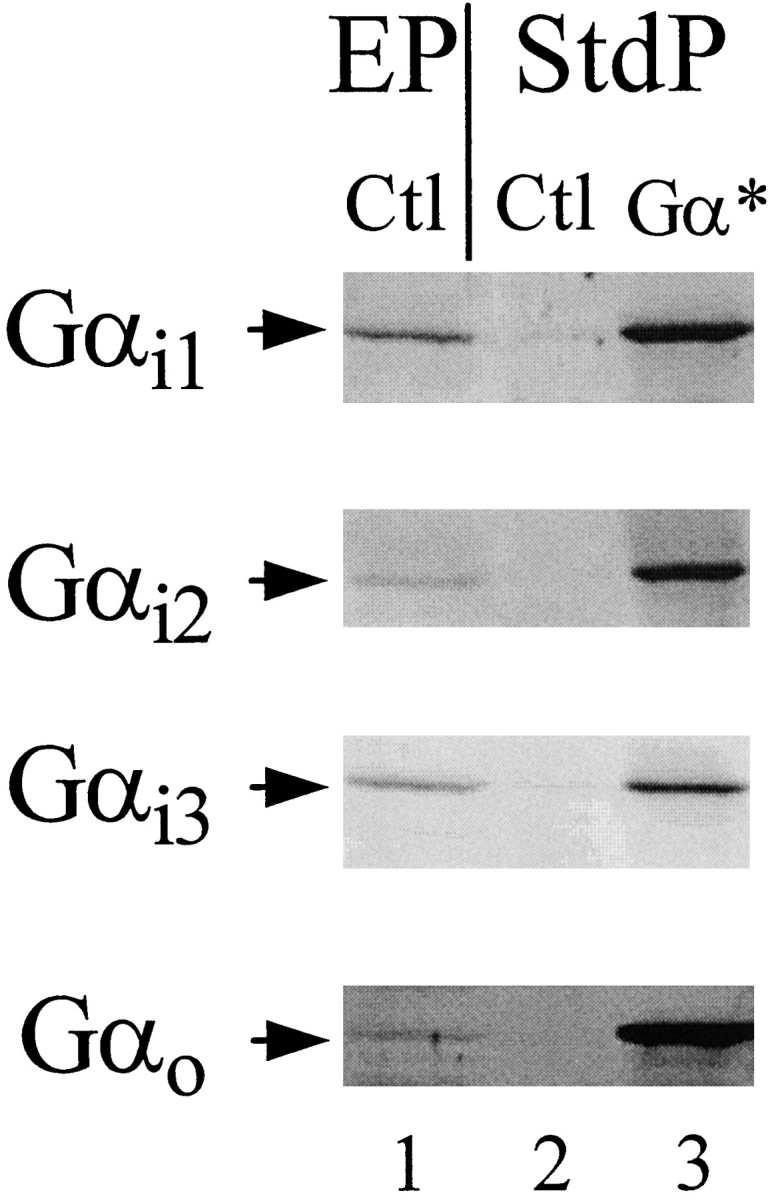
Expression of HSV-Gαi1*, -Gαi2*, -Gαi3*, and -Gαo* in NS20Y-D2L cell membranes. Lane 1 of each gel was loaded with 50 μg of control NS20Y-D2L cell membranes from an enriched membrane preparation (EP) as described in Materials and Methods. Lanes 2 and _3_were loaded with 10 μg of cell membranes from a standard membrane preparation (StdP) of control and HSV-Gαi1*, -Gαi2*-, -Gαi3*-, or -Gαo*-infected cells (18 hr), and Western analysis was completed using corresponding α subunit specific antibodies. The data shown are from a single experiment representative of three independent experiments.
Table 1.
Effect of HSV-Gα* subunits on GTPγS-stimulated cyclic AMP accumulation in membranes from PTX-treated NS20Y-D2Lcells
| Condition | cAMP accumulation (pmol·mg−1·min−1) | |||
|---|---|---|---|---|
| Basal | Forskolin (30 μm) | + GTPγS (1 μm) | Stimulation1-a (% of forskolin) | |
| Control | 8.8 ± 3.7 | 178 ± 39 | 355 ± 50* | 239 ± 60 |
| HSV-Gαo | 6.8 ± 2.1 | 186 ± 45 | 246 ± 71 | 134 ± 15 |
| HSV-Gαi1 | 11.6 ± 2.5 | 272 ± 70 | 259 ± 36 | 112 ± 19 |
| HSV-Gαi2 | 9.0 ± 1.5 | 292 ± 96 | 261 ± 63 | 118 ± 32 |
| HSV-Gαi3 | 10.0 ± 2.7 | 191 ± 43 | 197 ± 48 | 101 ± 3 |
| HSV-LacZ | 12.7 ± 2.3 | 175 ± 43 | 313 ± 53* | 200 ± 27 |
G-protein α subunit specificity for inhibition of forskolin-stimulated cAMP accumulation in NS20Y-D2Lcells
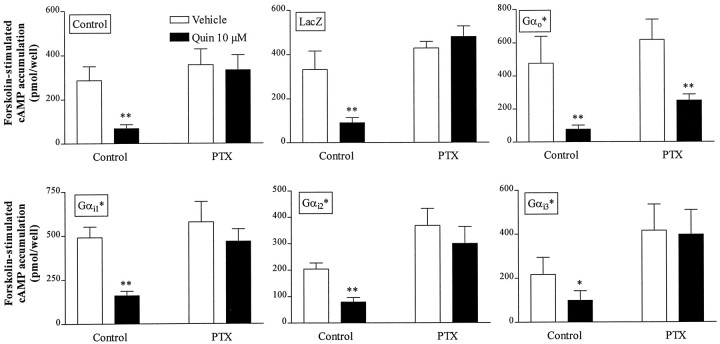
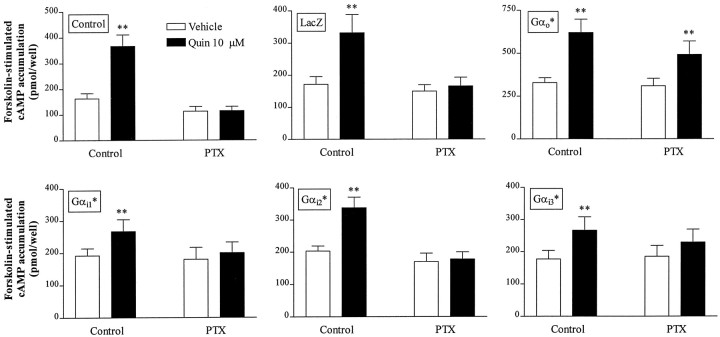
D2 receptor-mediated inhibition of forskolin-stimulated cAMP accumulation in several cell lines is blocked by previous PTX treatment, implicating PTX-sensitive Gα subunits (Neve et al., 1989; Watts and Neve, 1996). To identify the Gα subunit(s) involved in D2L receptor-mediated inhibition of cAMP accumulation in NS20Y-D2L cells, we infected cells with HSV recombinants expressing PTX-resistant Gα subunits Gαi1*, Gαi2*, Gαi3*, or Gαo*. Under these conditions, treatment with PTX eliminates coupling of D2L receptors to endogenous PTX-sensitive Gα subunits, but not D2L receptor coupling to heterologous PTX-resistant Gα subunits. In the absence of PTX treatment, quinpirole inhibited forskolin-stimulated cAMP accumulation in NS20Y-D2L cells infected with HSV-LacZ, -Gαi1*, -Gαi2*, -Gαi3*, or -Gαo*, whereas PTX pretreatment completely blocked quinpirole-induced inhibition of forskolin-stimulated cAMP accumulation in NS20Y-D2L cells and LacZ-infected NS20Y-D2Lcells (Fig. 5). PTX pretreatment also prevented quinpirole inhibition of cAMP accumulation in cells infected with Gαi1*, Gαi2*, or Gαi3*. In contrast, quinpirole inhibited cAMP accumulation by 57 ± 6% in PTX-treated cells that had been infected with HSV-Gαo*, compared with inhibition of 77 ± 3% in untreated and uninfected NS20Y- D2L cells, and 73 ± 2% in cells infected with HSV-LacZ but not treated with PTX.
Fig. 5.
Gα subunit specificity for inhibition of cAMP accumulation in NS20Y-D2L cells. Quinpirole-induced inhibition of forskolin-stimulated cAMP accumulation was examined in cells infected with PTX-resistant HSV-Gα subunits. NS20Y-D2L cells were untreated (Control), infected with HSV-LacZ, or infected with individual PTX-resistant Gα subunits for 18 hr. cAMP accumulation was stimulated with forskolin (10 μm) in the presence or absence of quinpirole (10 μm) for 15 min. Where indicated, cAMP accumulation was assessed after 6 hr treatment with 100 ng/ml pertussis toxin (PTX). Data shown are the mean ± SE of four or more independent experiments, assayed in duplicate. **p < 0.01, *p < 0.05 compared with forskolin alone (Student’s t test).
G-protein α subunit specificity for heterologous sensitization in NS20Y-D2L cells
D2L receptor-mediated heterologous sensitization of forskolin-stimulated cAMP accumulation in NS20Y-D2L cells is blocked by PTX treatment (Fig. 3). NS20Y-D2L cells were infected with HSV-Gα* recombinants and incubated with quinpirole to induce heterologous sensitization of forskolin-stimulated cAMP accumulation. In the absence of PTX, treatment with quinpirole (1 and 10 μm) for 2 hr potentiated forskolin-stimulated cAMP accumulation in control NS20Y-D2L cells and in NS20Y-D2L cells infected with HSV-LacZ, or any of the PTX-resistant mutants (Fig. 6) (data for 1 μm not shown). Specifically, cAMP accumulation was enhanced by 132 ± 19% in control cells, 90 ± 8% in HSV-LacZ cells, and 91 ± 22% (Gαo*), 67 ± 11% (Gαi2*), 52 ± 13% (Gαi3*), and 38 ± 8% (Gαi1*) in HSV-Gα*-infected cells. Although quinpirole-induced sensitization was significant in each condition, the magnitude of sensitization was reduced in cells infected with HSV-Gαi1*, -Gαi2*, and -Gαi3* (p < 0.05, compared with control cells; Dunnett’s post-repeated measures ANOVA). In some experiments, cells were initially treated with PTX (500 ng/ml) for 2 hr, followed by a 2 hr incubation with quinpirole in the presence of PTX (250 ng/ml). Consistent with the data presented in Figure 3, PTX pretreatment completely blocked quinpirole-induced sensitization of forskolin-stimulated cAMP accumulation in control and HSV-LacZ-infected NS20Y-D2L cells (Fig. 6). Rescue experiments with PTX-resistant Gα subunits revealed that quinpirole treatment produced heterologous sensitization in cells infected with Gαo* (57 ± 9%, n = 5) after PTX treatment. In contrast, infection with Gαi1*, Gαi2*, or Gαi3* failed to rescue sensitization in PTX-treated NS20Y-D2L cells. Similar results were seen in sensitization experiments with 1 μm quinpirole (data not shown).
Fig. 6.
Gα subunit specificity for heterologous sensitization in NS20Y-D2L cells. Quinpirole-induced sensitization of forskolin-stimulated cAMP accumulation was examined in cells infected with PTX-resistant HSV-Gα subunits. NS20Y-D2L cells were untreated (Control), infected with HSV-LacZ, or infected with individual PTX-resistant Gα subunits for 18 hr. NS20Y-D2L cells were incubated in the presence or absence of quinpirole (10 μm) for 2 hr and extensively washed, and cAMP accumulation was stimulated with forskolin (10 μm) for 15 min. Some experiments were completed after pertussis toxin (PTX) treatment. Cells were initially treated with PTX (500 ng/ml) for 2 hr, followed by a 2 hr incubation with quinpirole in the presence of PTX (250 ng/ml). Data shown are the mean ± SE of five to seven independent experiments, assayed in duplicate. **p < 0.01 compared with matched vehicle-treated cells (Student’s t test).
DISCUSSION
The complexity of the mechanisms by which a neurotransmitter, such as dopamine, modulates intracellular processes is increasingly evident because of our expanding knowledge of interactions among receptor subtypes, G-proteins, effectors, and other regulatory proteins. The molecular cloning of many signal transduction proteins makes it possible to study defined sets of signaling molecules in various cultured cells. In the current study, we transfected the D2L dopamine receptor into NS20Y neuroblastoma cells, which have many characteristics of striatal cholinergic cells (Amano et al., 1972).
Initial characterization of NS20Y-D2L cells demonstrated that acute activation of D2L receptors inhibited forskolin-stimulated cAMP accumulation. Furthermore, subacute (2 hr) activation of D2L receptors in NS20Y-D2L cells resulted in heterologous sensitization of adenylate cyclase, a phenomenon we previously characterized in the non-neuronal C6 glioma and HEK293 cell lines (Cox et al., 1995; Watts and Neve, 1996). D2L receptor-mediated inhibition and heterologous sensitization of forskolin-stimulated cAMP accumulation in NS20Y-D2L cells are PTX-sensitive; thus, these cells provide a neuronal-like model system with which to examine the functional coupling specificity for D2Lreceptor–Gαi/o signaling events. This was accomplished by viral (HSV)-mediated gene delivery of cDNAs encoding PTX-resistant G-protein α subunits. The use of mutant PTX-resistant G-proteins is a powerful technique that has been used successfully to study G-protein coupling to Ca2+ currents (Taussig et al., 1992) and D2 receptor-G-protein coupling to inhibition of cAMP accumulation in other cell types (Senogles, 1994; O’Hara et al., 1996). In the current study, HSV-mediated expression of each of the Gα* subunits produced functional expression of the subunits in NS20Y-D2L cells (Table 1). The expression of each Gα* subunit was robust (Fig. 4), and because all studies were performed in one NS20Y-D2L cell line, confounds attributable to clonal variation in the expression level of D2 receptors and endogenous components of the signaling pathways, such as G-proteins and adenylate cyclase, are likely to be minimal (Mullaney et al., 1995;Watts et al., 1995b; Kenakin, 1997). Furthermore, the short duration of expression of the PTX-resistant Gα subunits is likely to diminish adaptive changes in cellular signaling and growth processes that may occur as a result of chronic expression of exogenous G-protein α subunits (Gordeladze et al., 1997).
Heterologous sensitization induced by Gαi/o-coupled receptors is blocked by PTX, but no studies have examined the G-protein specificity for this neuroadaptive mechanism, which could be mediated by a G-protein distinct from that mediating inhibition of adenylate cyclase (Watts and Neve, 1996; Watts et al., 1998). However, using PTX-insensitive G-proteins, we found that selective activation of Gαo* by D2L receptors mediated both inhibition and heterologous sensitization of forskolin-stimulated cAMP accumulation in NS20Y-D2L cells. Expression of mutant Gαi1*, Gαi2*, and Gαi3* subunits did not rescue inhibition or sensitization in PTX-treated cells. Moreover, in the absence of PTX treatment, expression of each of the mutant Gαi subunits appeared to reduce the magnitude of sensitization compared with control NS20Y-D2L cells. This effect may be attributable to some degree of constitutive inhibition of adenylate cyclase by recombinant Gαisubunits. Alternatively, the reduction in D2Lreceptor-mediated sensitization caused by expression of the Gαi* subunits could be attributable to sequestration of βγ subunits, because βγ sequestration by expression of Gαt or the C terminus of β-adrenergic receptor kinase can prevent heterologous sensitization (Avidor-Reiss et al., 1996;Thomas and Hoffman, 1996).
Although the exact mechanism responsible for heterologous sensitization remains to be elucidated, we propose that persistent activation of Gαo-linked receptors leads to enhanced Gαs-adenylate cyclase coupling, possibly through a βγ subunit-dependent event (Avidor-Reiss et al., 1996; Thomas and Hoffman, 1996). This hypothesis is based in part on observations that heterologous sensitization of adenylate cyclase is associated with an increase in the number of [3H]forskolin-labeled sites (Jones and Bylund, 1990) and decreased palmitoylated Gαs (Ammer and Schulz, 1997), which could lead to increased adenylate cyclase activity. Increased Gαs–adenylate cyclase interactions have also been proposed as a mechanism for sensitization of adenylate cyclase by antidepressant treatment (Chen and Rasenick, 1995). Our own data demonstrating that activation of D2 receptors sensitizes multiple forms of Gαs-activated adenylate cyclase add further support to this hypothesis (Watts and Neve, 1996).
We recently described mechanistic differences between short- and long-term sensitization by D4 dopamine receptors in HEK293 cells (Watts et al., 1998). Long-term (18 hr) agonist treatment of HEK-D4 cells results in a greater magnitude of sensitization in both intact cells and cell membranes compared with short-term agonist treatment (2 hr). Long-term sensitization, but not short-term sensitization, is accompanied by decreased immunoreactivity of Gαi in membranes. A reduction of Gαi is likely to enhance adenylate cyclase activity and suggests that, in addition to increased Gαs-adenylate cyclase interactions, long-term sensitization involves other mechanisms. In light of the present results, it would be particularly interesting to examine Gαo levels in membranes after short- and long-term agonist exposure in NS20Y-D2L cells. Moreover, the current observation that the selective stimulation of Gαo was responsible for both the acute (inhibition) and subacute (sensitization) effects of D2L receptor activation in NS20Y-D2L cells provides an impetus to examine the Gα subunit specificity involved in long-term heterologous sensitization.
Selective coupling of D2 receptors to Gαo has been shown using other approaches in both primary cultures of pituitary cells and pituitary-derived cell lines. Specifically, antiserum to Gαo, but not Gαi1/2 or Gαi3, blocks D2 receptor coupling to Ca2+ channels in rat anterior pituitary cells (Lledo et al., 1992). Antisense depletion of Gαo in pituitary GH4C1 cells revealed that activation of Gαo is largely responsible for D2-mediated inhibition of Ca2+ entry (Liu et al., 1994). In contrast, studies of cAMP metabolism suggested that selective activation of Gαi subunits is responsible for the D2-mediated inhibition of cAMP accumulation. For example,Liu et al. (1994) demonstrated that antisense reduction of Gαo does not alter D2 receptor-mediated inhibition of cAMP accumulation, whereas reduction of Gαi2 blocks this response in GH4C1 cells. Using ZnSO4-inducible expression of PTX-resistant Gα subunits in GH4C1 cells, Senogles (1994) reported that D2L receptors couple to Gαi3 to inhibit cAMP accumulation, whereas D2S receptors couple to Gαi2 for this signaling pathway. In 7315c pituitary cells, antiserum directed against Gαi1/2 blunts D2-mediated inhibition of adenylate cyclase, but antisera to Gαi3, Gαo, Gαs, or Gαq do not (Izenwasser and Côté, 1995). Although methodological differences (antisense, antisera, and inducible expression of Gα mutants) cannot be ruled out, the results of the current study and those discussed above suggest that there may be divergent G-protein specificity patterns for D2 receptor signaling in pituitary versus neuronal-like cells.
A recent study using D2 receptors and PTX-insensitive G-proteins both stably expressed in CCL1.3 fibroblast cells found that D2 receptor activation inhibits cAMP accumulation through Gαi2 and Gαi3 but not Gαi1 or Gαo (O’Hara et al., 1996). This same group also examined D2L receptor-G-protein specificity in a neuronal-like cell line, MN9D cells. As in CCL1.3 cells, D2 receptors inhibit cAMP accumulation through Gαi2 but not Gαoin MN9D cells. Although the reason for the differences between the study completed in MN9D cells and the current results in NS20Y cells is unclear, it may reflect differences in the endogenous signaling proteins, such as subtypes of adenylate cyclase that are differentially sensitive to Gαo (Taussig et al., 1994) or subtypes of G-protein βγ subunits that differentially support an interaction between D2L and Gαo. Previous work supports a role for Gαo in adenylate cyclase inhibition by muscarinic (Migeon et al., 1995), somatostatin (Murthy et al., 1996), and opiate (Murthy and Makhlouf, 1996) receptors. Interestingly, our preliminary data indicate that Gαo can also mediate both inhibition and heterologous sensitization of adenylate cyclase in HEK293 cells, which do not express endogenous Gαo(B. L. Wiens, V. J. Watts, K. A. Neve, unpublished observations).
In the current study we have analyzed the selective activation of PTX-sensitive G-proteins by D2L dopamine receptors in a neuronal-like environment. We have demonstrated the utility of the HSV expression vector for examination of recombinant signaling proteins. Overnight infection with PTX-resistant Gα subunits resulted in marked increases in protein expression as assessed by Western blotting. The function and PTX resistance of individual G-protein α subunits was confirmed by examining the ability of Gαi1*, Gαi2*, Gαi3*, or Gαo* to inhibit GTPγS-stimulated cAMP accumulation after PTX treatment. Under these conditions, only Gαo* demonstrated functional coupling to D2L dopamine receptors expressed in NS20Y cells. D2L receptor coupling to Gαo* was confirmed for two PTX-sensitive signaling events, one in which the acute response of D2L receptor activation is measured and a second that requires more prolonged agonist occupation of the receptor. These are novel findings in neuronal-like cells and are particularly striking when one considers that two separate signaling events resulting in opposing changes in adenylate cyclase activity are both mediated by the same class of Gα subunits. Heterologous sensitization of adenylate cyclase may be one neuroadaptive mechanism by which a single protein contributes to maintenance of cellular homeostasis during a chronic inhibitory signal.
Footnotes
This work was supported by Grant MH45372 from the U.S. Public Health Service, the Veterans Affairs Merit Review and Research Career Scientist Programs, and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression. We thank Dr. Amy Eshleman for careful reading of this manuscript.
Correspondence should be addressed to Dr. Kim A. Neve, Medical Research Service (R&D-30), Veterans Affairs Medical Center, 3710 SW U.S. Veterans Hospital Road, Portland, OR 97201.
Dr. Watts’ present address: Department of Medicinal Chemistry and Molecular Pharmacology, Purdue University, West Lafayette, IN 47907.
REFERENCES
- 1.Amano T, Richelson E, Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones. Proc Natl Acad Sci USA. 1972;69:258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammer H, Schulz R. Morphine dependence in human neuroblastoma SH-SY5Y cells is associated with adaptive changes in both the quantity and functional interaction of PGE1 receptors and stimulatory G proteins. Brain Res. 1996;707:235–244. doi: 10.1016/0006-8993(95)01265-6. [DOI] [PubMed] [Google Scholar]
- 3.Ammer H, Schulz R. Enhanced stimulatory adenylyl cyclase signaling during opioid dependence is associated with a reduction in palmitoylated Gsα. Mol Pharmacol. 1997;52:993–999. doi: 10.1124/mol.52.6.993. [DOI] [PubMed] [Google Scholar]
- 4.Avidor-Reiss T, Nevo I, Levy R, Pfeuffer T, Vogel Z. Chronic opioid treatment induces adenylyl cyclase V superactivation: involvement of Gβγ. J Biol Chem. 1996;271:21309–21315. doi: 10.1074/jbc.271.35.21309. [DOI] [PubMed] [Google Scholar]
- 5.Bates MD, Senogles SE, Bunzow JR, Liggett SB, Civelli O, Caron MG. Regulation of responsiveness at D2 dopamine receptors by receptor desensitization and adenylyl cyclase sensitization. Mol Pharmacol. 1991;39:55–63. [PubMed] [Google Scholar]
- 6.Chen J, Rasenick MM. Chronic antidepressant treatment facilitates G protein activation of adenylyl cyclase without altering G protein content. J Pharmacol Exp Ther. 1995;275:509–517. [PubMed] [Google Scholar]
- 7.Cox BA, Henningsen RA, Spanoyannis A, Neve RL, Neve KA. Contributions of conserved serine residues to the interactions of ligands with dopamine D2 receptors. J Neurochem. 1992;59:627–635. doi: 10.1111/j.1471-4159.1992.tb09416.x. [DOI] [PubMed] [Google Scholar]
- 8.Cox BA, Rosser MP, Kozlowski MR, Duwe KM, Neve RL, Neve KA. Regulation and functional characterization of a rat recombinant dopamine D3 receptor. Synapse. 1995;21:1–9. doi: 10.1002/syn.890210102. [DOI] [PubMed] [Google Scholar]
- 9.Gordeladze JO, Hovik KE, Merendino JJ, Hermouet S, Gutkind S, Accili D. Effect of activating and inactivating mutations of Gs- and Gi2-alpha protein subunits on growth and differentiation of 3T3–L1 preadipocytes. J Cell Biochem. 1997;64:242–257. doi: 10.1002/(sici)1097-4644(199702)64:2<242::aid-jcb8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Huff RM. Signaling pathways modulated by dopamine receptors. In: Neve KA, Neve RL, editors. The dopamine receptors. Humana; Totowa, NJ: 1997. pp. 167–192. [Google Scholar]
- 11.Izenwasser S, Côté TE. Inhibition of adenylyl cyclase activity by a homogeneous population of dopamine receptors: selective blockade by antisera directed against Gi1 and/or G i2. J Neurochem. 1995;64:1614–1621. doi: 10.1046/j.1471-4159.1995.64041614.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones SB, Bylund DB. Effects of α2-adrenergic agonist preincubation on subsequent forskolin-stimulated adenylate cyclase activity and [3H]forskolin binding in membranes from HT29 cells. Biochem Pharmacol. 1990;40:871–877. doi: 10.1016/0006-2952(90)90329-j. [DOI] [PubMed] [Google Scholar]
- 13.Kenakin T. Differences between natural and recombinant G protein-coupled receptor systems with varying receptor G protein stoichiometry. Trends Pharmacol Sci. 1997;18:456–464. doi: 10.1016/s0165-6147(97)01136-x. [DOI] [PubMed] [Google Scholar]
- 14.Liu YF, Jakobs KH, Rasenick MM, Albert PR. G protein specificity in receptor-effector coupling: analysis of the roles of Go and Gi2 in GH4C1 pituitary cells. J Biol Chem. 1994;269:13880–13886. [PubMed] [Google Scholar]
- 15.Lledo PM, Homburger V, Bockaert J, Vincent J-D. Differential G protein-mediated coupling of D2 dopamine receptors to K+ and Ca2+ currents in rat anterior pituitary cells. Neuron. 1992;8:455–463. doi: 10.1016/0896-6273(92)90273-g. [DOI] [PubMed] [Google Scholar]
- 16.Migeon JC, Thomas SL, Nathanson NM. Differential coupling of m2 and m4 muscarinic receptors to inhibition of adenylyl cyclase by Giα and Goα subunits. J Biol Chem. 1995;70:16070–16074. doi: 10.1074/jbc.270.27.16070. [DOI] [PubMed] [Google Scholar]
- 17.Monsma FJ, Brassard DL, Sibley DR. Identification and characterization of D1 and D2 dopamine receptors in cultured neuroblastoma and retinoblastoma clonal cell lines. Brain Res. 1989;492:314–324. doi: 10.1016/0006-8993(89)90915-3. [DOI] [PubMed] [Google Scholar]
- 18.Mullaney I, Shah BH, Wise A, Milligan G. Expression of the human β2-adrenoceptor in NCB20 cells results in agonist activation of adenylyl cyclase and agonist-mediated selective down-regulation of Gsα. J Neurochem. 1995;65:545–553. doi: 10.1046/j.1471-4159.1995.65020545.x. [DOI] [PubMed] [Google Scholar]
- 19.Murthy KS, Makhlouf GM. Opioid μ, δ, and κ receptor-induced activation of phospholipase C-β3 and inhibition of adenylyl cyclase is mediated by Gi2 and Go in smooth muscle. Mol Pharmacol. 1996;50:870–877. [PubMed] [Google Scholar]
- 20.Murthy KS, Coy DH, Makhlouf GM. Somatostatin receptor-mediated signaling in smooth muscle: activation of phospholipase C-β3 by Gβγ and inhibition of adenylyl cyclase by Gαi1 and Gαo. J Biol Chem. 1996;271:23458–23463. doi: 10.1074/jbc.271.38.23458. [DOI] [PubMed] [Google Scholar]
- 21.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–62. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 22.Neve KA, Henningsen RA, Bunzow JR, Civelli O. Functional characterization of a rat dopamine D-2 receptor cDNA expressed in a mammalian cell line. Mol Pharmacol. 1989;36:446–451. [PubMed] [Google Scholar]
- 23.Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79:435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- 24.Nordstedt C, Fredholm BB. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Anal Biochem. 1990;189:231–234. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 25.O’Hara CM, Tang L, Taussig R, Todd RD, O’Malley KL. Dopamine D2L receptor couples to Gαi2 and Gαi3 but not Gαi1, leading to the inhibition of adenylate cyclase in transfected cell lines. J Pharmacol Exp Ther. 1996;278:354–360. [PubMed] [Google Scholar]
- 26.Senogles SE. The D2 dopamine receptor isoforms signal through distinct Giα proteins to inhibit adenylyl cyclase: a study with site-directed mutant Giα proteins. J Biol Chem. 1994;269:23120–23127. [PubMed] [Google Scholar]
- 27.Sharma SK, Klee WA, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci USA. 1975;72:3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starr S, Kozell LB, Neve KA. Drug-induced proliferation of dopamine D2 receptors on cultured cells. J Neurochem. 1995;65:569–577. doi: 10.1046/j.1471-4159.1995.65020569.x. [DOI] [PubMed] [Google Scholar]
- 29.Taussig R, Sanchez S, Rifo M, Gilman AG. Inhibition of the ω-conotoxin-sensitive calcium current by distinct G proteins. Neuron. 1992;8:799–809. doi: 10.1016/0896-6273(92)90100-r. [DOI] [PubMed] [Google Scholar]
- 30.Taussig R, Tang W-J, Hepler JR, Gilman AG. Distinct patterns of bidirectional regulation of mammalian adenylyl cyclases. J Biol Chem. 1994;269:6093–6100. [PubMed] [Google Scholar]
- 31.Thomas JM, Hoffman BB. Isoform-specific sensitization of adenylyl cyclase activity by prior activation of inhibitory receptors: role of βγ subunits in transducing enhanced activity of the type VI isoform. Mol Pharmacol. 1996;49:907–914. [PubMed] [Google Scholar]
- 32.Watts VJ, Neve KA. Sensitization of endogenous and recombinant adenylate cyclase by activation of D2 dopamine receptors. Mol Pharmacol. 1996;50:966–976. [PubMed] [Google Scholar]
- 33.Watts VJ, Neve KA. Activation of type II adenylate cyclase by D2 and D4 but not D3 dopamine receptors. Mol Pharmacol. 1997;52:181–186. doi: 10.1124/mol.52.2.181. [DOI] [PubMed] [Google Scholar]
- 34.Watts VJ, Lawler CP, Fox DR, Neve KA, Nichols DE, Mailman RB. LSD and structural analogs: pharmacological evaluation at D1 dopamine receptors. Psychopharmacology (Berl) 1995a;118:401–409. doi: 10.1007/BF02245940. [DOI] [PubMed] [Google Scholar]
- 35.Watts VJ, Lawler CP, Gonzales AJ, Zhou QY, Civelli O, Nichols DE, Mailman RB. Spare receptors and intrinsic activity: studies with D1 dopamine receptor agonists. Synapse. 1995b;21:177–187. doi: 10.1002/syn.890210211. [DOI] [PubMed] [Google Scholar]
- 36.Watts VJ, Vu MN, Wiens BL, Jovanovic V, Van Tol HHM, Neve KA (1998) Short- and long-term heterologous sensitization of adenylate cyclase by D4 dopamine receptors. Psychopharmacology, in press. [DOI] [PubMed]