Blockade of a Retinal cGMP-gated Channel by Polyamines (original) (raw)
Abstract
The cyclic nucleotide–gated (CNG) channel in retinal rods converts the light-regulated intracellular cGMP concentration to various levels of membrane potential. Blockade of the channel by cations such as Ca2+ and Mg2+ lowers its effective conductance. Consequently, the membrane potential has very low noise, which enables rods to detect light with extremely high sensitivity. Here, we report that three polyamines (putrescine, spermidine, and spermine), which exist in both the intracellular and extracellular media, also effectively block the CNG channel from both sides of the membrane. Among them, spermine has the greatest potency. Extracellular spermine blocks the channel as a permeant blocker, whereas intracellular spermine appears to block the channel in two conformations—one permeant, and the other non- (or much less) permeant. The membrane potential in rods is typically depolarized to approximately −40 mV in the dark. At this voltage, K 1/2 of the CNG channel for extracellular spermine is 3 μM, which is 100–1,000-fold higher affinity than that of the NMDA receptor-channel for extracellular spermine. Blockade of the CNG channel by polyamines may play an important role in suppressing noise in the signal transduction system in rods.
Keywords: cyclic nucleotide–gated channel, ion permeation, putrescine, spermidine, spermine
introduction
Cyclic nucleotide–gated (CNG)1 channels mediate visual signal transduction in vertebrate retinal rods (Yau and Baylor, 1989). The channel opens when intracellular cGMP concentration is elevated in darkness, and closes when cGMP concentration is reduced in light. An open CNG channel conducts Na+ and K+ almost equally well and consequently has a reversal potential near 0 mV (Werblin, 1975; Bader et al., 1979; Yau et al., 1981; Bastian and Fain, 1982; Woodruff et al., 1982; Capovilla et al., 1983; Hodgkin et al., 1984, 1985; Yau and Nakatani, 1984; Bododia and Detwiler, 1985; Baylor and Nunn, 1986). Activation of the channel by cGMP depolarizes the plasma membrane, whereas deactivation hyperpolarizes it. The CNG channel functions as a chemoelectrical signal converter: it converts the light-regulated intracellular cGMP concentration into various levels of membrane potential.
The CNG channel also passes Ca2+ and Mg2+ (Capovilla et al., 1983; Hodgkin et al., 1985; Torre et al., 1987; Nakatani and Yau, 1988). The Ca2+ influx through the CNG channel influences light adaptation, which is critical for the retinal rod's broad dynamic range of light sensitivity (Kaupp and Koch, 1992; Koutalos and Yau, 1993). Permeation rates of Ca2+ and Mg2+ are considerably lower than those of Na+ and K+. Therefore, Ca2+ and Mg2+ act as permeant blockers of the CNG channel (Haynes et al., 1986; Stern et al., 1987; Colamartino et al., 1991; Zimmerman and Baylor, 1992; Root and MacKinnon, 1993; Eismann et al., 1994; Park and MacKinnon, 1995). Blockade of the channel by divalent cations dramatically reduces the effective conductance of the channel. Consequently, membrane potential noise in rods is very low, which enables them to detect light with exceedingly high sensitivity (Yau and Baylor, 1989).
Like the CNG channel, inward-rectifier K+ channels and some glutamate-gated channels are also blocked by divalent cations (Ault et al., 1980; Mayer et al., 1984; Nowak et al., 1984; Horie et al., 1987; Matsuda et al., 1987; Vandenberg, 1987). These two classes of channels were recently shown to be blocked by polycationic polyamines, ubiquitous intracellular constituents that also exist in extracellular media (Rock and Macdonald, 1992a,b; Araneda et al., 1993; Benveniste and Mayer, 1993; Ficker et al., 1994; Lopatin et al., 1994; Bowie and Mayer, 1995; Donevan and Rogawski, 1995; Igarashi and Williams, 1995; Fakler et al., 1995; Isa et al., 1995; Koh et al., 1995; Kamboj et al., 1995; Bahring et al., 1997; Williams, 1997). Polyamines block the pore of inward-rectifier K+ channels from the intracellular side. They block some glutamate-gated channels from both the intracellular and the extracellular sides in a permeant manner. Motivated by these recent findings, we examined the effects of polyamines on a CNG channel. The channel was heterologously expressed in Xenopus oocytes by direct injection of RNA that encodes the α subunit of the bovine retinal cyclic nucleotide-gated channel (Kaupp et al., 1989). We found that three polyamines (spermine, spermidine, and putrescine) blocked the channel from both the intracellular and extracellular sides. The mechanisms by which polyamines block the CNG channel are complex.
methods
Molecular Biology and Oocyte Preparation
The cDNA of bovine rod cGMP-gated channel α subunit cloned into pGEM-HE plasmid was kindly provided by Dr. Steven Siegelbaum (Kaupp et al., 1989; Goulding et al., 1992; Liman et al., 1992). RNA was synthesized using T7 polymerase (Promega Corp.) from Nhe1-linearized cDNA. Oocytes harvested from Xenopus laevis (Xenopus One) were incubated in a solution containing (mM): 82.5 NaCl, 2.5 KCl, 1.0 MgCl2, 5.0 HEPES, pH 7.6, and 2–4 mg/ml collagenase. The oocyte preparation was agitated using a platform shaker (80 rpm) for 60–90 min. It was then rinsed thoroughly and stored in a solution containing (mM) 96 NaCl, 2.5 KCl, 1.8 CaCl2, 1.0 MgCl2, 5 HEPES, pH 7.6, and 50 μM gentamicin. Defolliculated oocytes were selected and injected with RNA at least 2 and 16 h after collagenase treatment, respectively. All oocytes were stored in an incubator at 18°C.
Patch Recording and Solutions
The CNG channel currents were recorded in either the inside-out or the outside-out configuration from Xenopus oocytes (injected with the CNG-channel cRNA) with an Axopatch 200B amplifier (Axon Instruments, Inc.). The recorded signal was filtered at 1 kHz and sampled at 5 kHz using an analogue-to-digital converter (DigiData 1200; Axon Instruments, Inc.) interfaced with a personal computer. pClamp6 software (Axon Instruments, Inc.) was used to control the amplifier and acquire the data. Macroscopic current–voltage curves were recorded as membrane voltage was linearly ramped (25–50 mV/s). For the inside-out configuration, the currents obtained in the absence of cGMP were used as templates for subsequent off-line background current corrections. For the outside-out configuration, currents at −80 mV in the presence of 20 mM extracellular Mg2+ were used to estimate the background currents. During background current corrections, the background currents were assumed to vary linearly as a function of membrane voltage between −80 and +80 mV.
Both the internal and external solutions contained (mM): 130 NaCl, 0.5 EDTA, and 5 HEPES, pH 7.6. To activate the channel, 1 mM (a near-saturating concentration) cGMP was included in the internal solution. Polyamine-containing internal and external solutions were prepared daily.
results
Blockade of the CNG Channel by Extracellular Polyamines
Fig. 1 A shows macroscopic current–voltage (I–V) relations of the CNG channel recorded in the outside-out configuration. Both sides of the membrane were exposed to 130 mM Na+ and 0.5 mM EDTA. To activate the channel, a near saturating concentration of cGMP (1 mM) was included in the pipette solution. In the absence of blocking ions, the I–V relation of the CNG channel was nearly linear, with very slight outward rectification. We used the I–V relation in the presence of 1 μM extracellular spermine to illustrate the basic properties of channel blockade. Between 50 and 80 mV, the I–V curves with and without spermine were superimposed, indicating that in this voltage range spermine had no effect. Below 50 mV, spermine blocked the channel and the extent of blockade increased when membrane voltage was reduced, as manifested by a gradual divergence of the two I–V curves. However, contrary to expectation for a typical ionic pore blocker, channel blockade by spermine was relieved (rather than enhanced) at extreme negative membrane voltage, as manifested by a reconvergence of the I–V curves at negative voltages.
Figure 1.
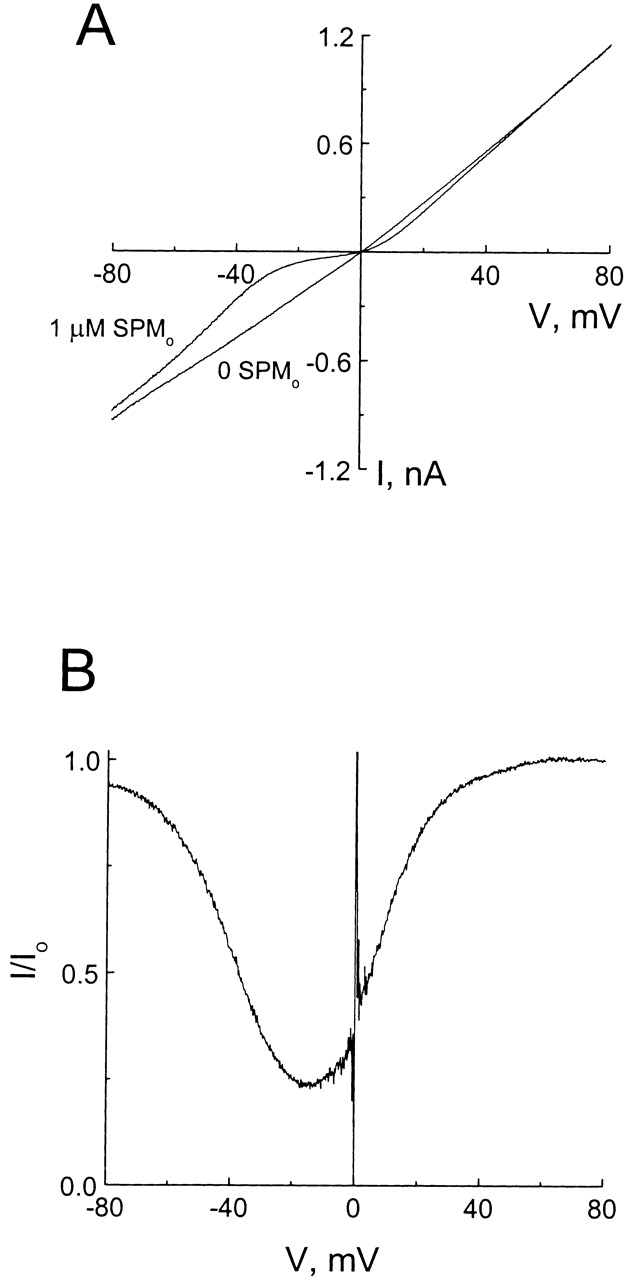
Blockade of the CNG channel by extracellular spermine. (A) Macroscopic current–voltage relations (I–V curves) of the CNG channel in the absence and presence of 1 μM extracellular spermine. (B) The fraction of unblocked current (I/Io), taken from A, is plotted against membrane voltage.
In Fig. 1 B, the fraction of unblocked current in the presence of 1 μM extracellular spermine (I/Io) is plotted against membrane voltage. The curve has an inverted bell shape with maximal inhibition at −15 mV: channel blockade was enhanced when positive membrane voltage was lowered, and relieved again at large negative voltages. Extracellular spermine effectively caused the channel to conduct in a bidirectionally rectifying manner. This bimodal character of channel blockade as a function of voltage is commonly viewed as the hallmark of a permeant ionic pore blocker, and is explained as follows: hyperpolarization electrostatically enhances the binding of positively charged blockers into the pore; however, with sufficient hyperpolarization, the excessive transmembrane electrical force “drives” permeant blockers through the pore, which results in relief of blockade.
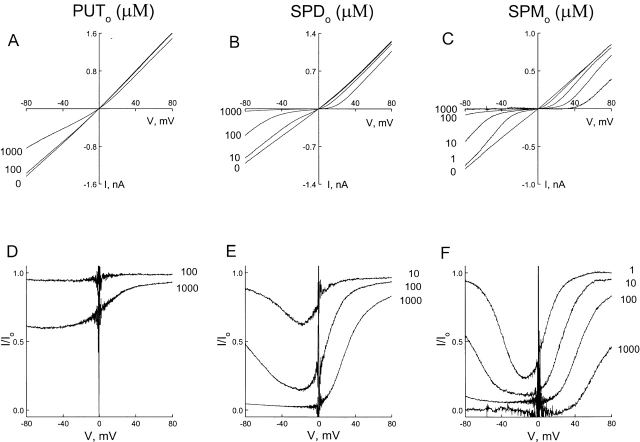
Fig. 2, A–C, shows CNG channel I–V relations in the absence and presence of various concentrations of extracellular putrescine, spermidine, and spermine, respectively. The fraction of unblocked currents is plotted against membrane voltage in D–F. All three polyamines exhibited qualitatively similar behaviors, predominantly blocking in a voltage-dependent manner at positive voltages and becoming permeant at negative voltages. The blocking phase of the curves for spermine and spermidine was similar but steeper than that for putrescine. Interestingly, steepness of the relief phases of the curve correlated qualitatively with the valence of the polyamine. (Putrescine, spermidine, and spermine carry up to two, three, and four positive charges, respectively.)
Figure 2.
Comparison of CNG channel blockade by three extracellular polyamines. (A–C) Macroscopic I–V curves of the CNG channel in the absence and presence of various concentrations of extracellular putrescine (PUT), spermidine (SPD), and spermine (SPM). (D–F) The corresponding fraction of unblocked currents is plotted against membrane voltage.
As shown in Fig. 2, current at negative voltages was progressively inhibited by increasing polyamine concentrations, and essentially vanished in the presence of 1 mM spermine. Therefore, although current enhancement at negative voltages resulted from polyamine permeation, Na+ still carried nearly all the current.
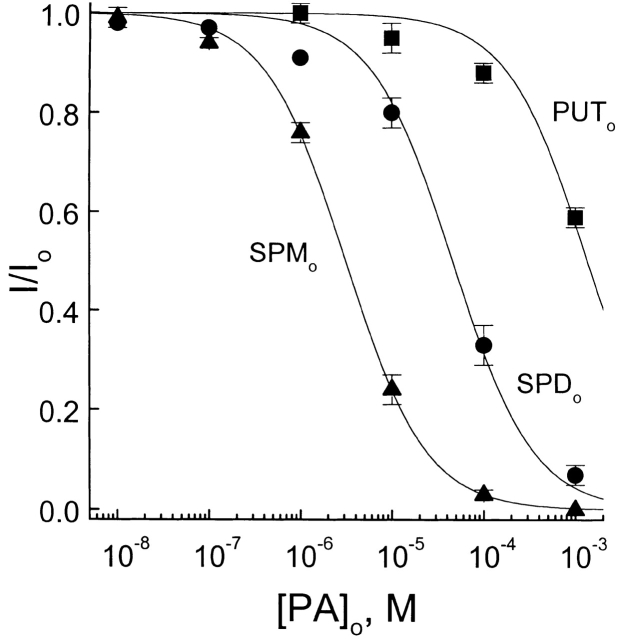
Because the membrane potential in rods is depolarized to about −40 mV in darkness, we plotted the fraction of unblocked currents of the CNG channel at −40 mV against polyamine concentration and fitted the data with hyperbolic functions in Fig. 3. The least-squares estimates of K 1/2 for putrescine, spermidine, and spermine at −40 mV were 1.4 mM, 50 μM, and 3.1 μM, respectively.
Figure 3.
Comparative potency of the three extracellular polyamines. The fraction of unblocked currents at −40 mV is plotted against the concentrations of putrescine, spermidine, and spermine. The solid curves are least-squares fits of equations of the form I/Io = PA K 1/2/(PA K 1/2 + [PA]), where PA K 1/2 is the concentration at which a given polyamine blocks half the current through the CNG channel, and [PA] is the concentration of the polyamine. The K 1/2 values for extracellular putrescine, spermidine, and spermine are 1.4 mM, 50 μM, and 3.1 μM, respectively.
Blockade of the CNG Channel by Intracellular Polyamines
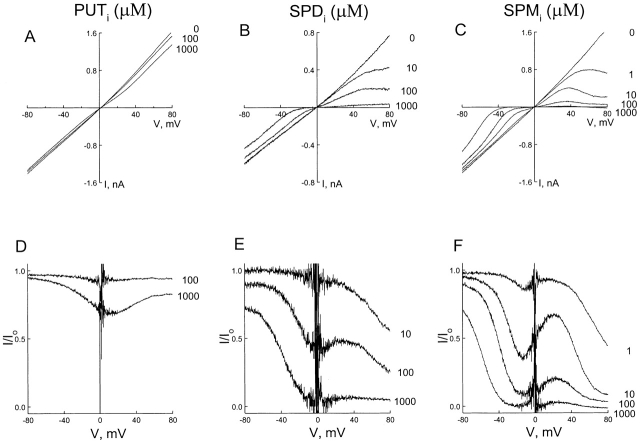
Fig. 4 A shows macroscopic I–V relations recorded in the inside-out configuration, with 130 mM Na+ and 0.5 mM EDTA on both sides of the membrane. To activate the channel, 1 mM cGMP was included in the bath solution. We used the I–V curve in the presence of 10 μM intracellular spermine to illustrate the basic properties of channel blockade. At negative voltages, the shape of the I–V curve with intracellular spermine was very similar to that with extracellular spermine (compare Figs. 1 A and 4 A). Inhibition was stronger near zero than at negative voltages, consistent with spermine binding within the transmembrane electrical field. At positive voltages, the I–V curve was complex. If intracellular spermine, like extracellular spermine, simply acted as a typical permeant blocker, one would expect relief of blockade at positive voltages. That is, the I–V curves with and without spermine would converge at positive voltages. However, the I–V curves diverge dramatically at positive voltages, suggesting that intracellular spermine did not merely (or at all) act as a permeant ionic blocker.
Figure 4.
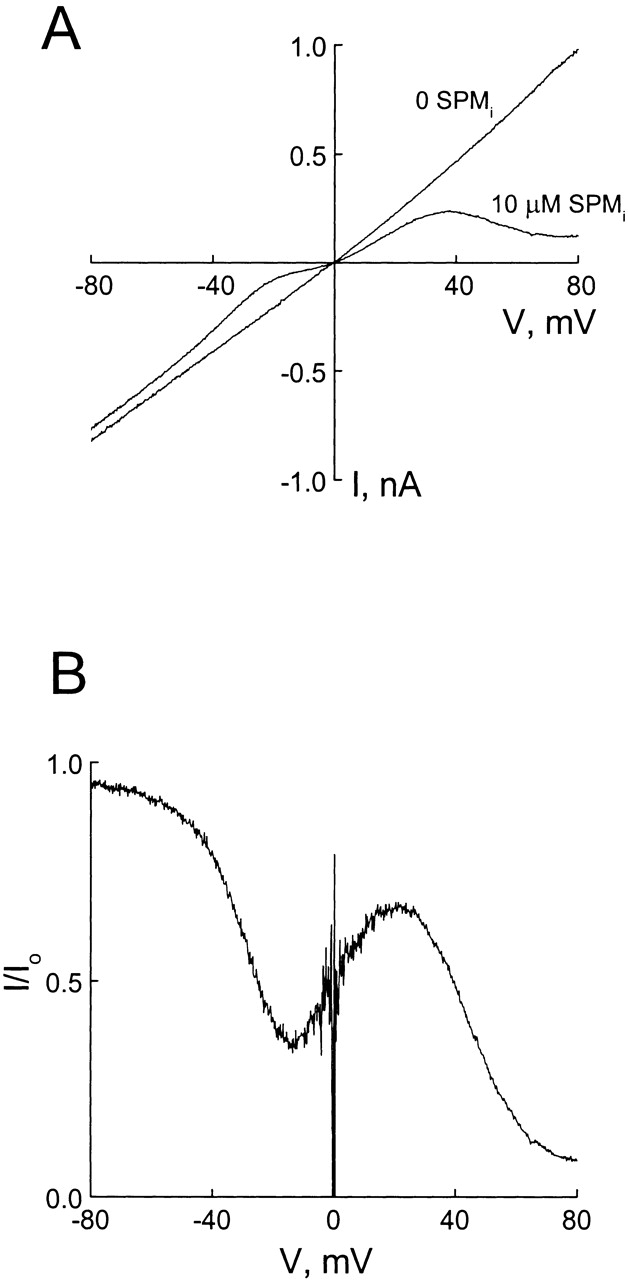
Blockade of the CNG channel by 10 μM intracellular spermine. (A) Macroscopic I–V curves of the CNG channel in the absence and presence of spermine. (B) The fraction of unblocked current, taken from A, is plotted against membrane voltage.
The fraction of unblocked current in the presence of 10 μM intracellular spermine is plotted against membrane voltage in Fig. 4 B. The curve displayed both a minimum and a maximum. Interestingly, the minima for both intracellular and extracellular spermine occur at about −15 mV (compare Figs. 1 B and 4 B).
Fig. 5, A–C, shows I–V relations for the CNG channel in the absence and presence of various concentrations of intracellular putrescine, spermidine, and spermine, respectively. The fraction of unblocked currents is plotted against membrane voltage in D–F. As shown in Fig. 5 F, the positive slope between the minimum and maximum varied with spermine concentration, and essentially disappeared when the concentration reached 1 mM. In the case of spermidine, the minimum and maximum were not as well defined as for spermine, almost fusing together to form a plateau (Fig. 5 E). For putrescine, the curve appeared to reach a plateau just below 80 mV.
Figure 5.
CNG channel blockade by three intracellular polyamines. (A–C) Macroscopic I–V curves of the CNG channel in the absence and the presence of various concentrations of intracellular putrescine, spermidine, and spermine. (D–F) The fraction of unblocked currents, taken from A–C, is plotted against membrane voltage.
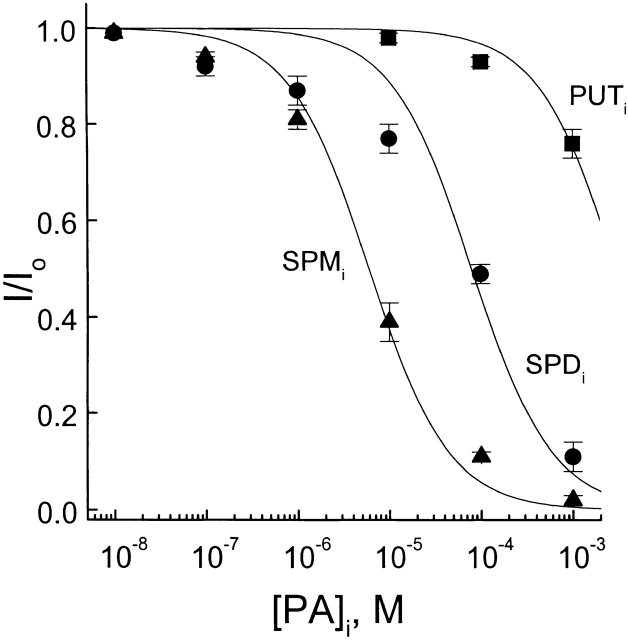
In Fig. 6, the fraction of unblocked currents of the CNG channel at +40 mV is plotted against the concentration of three intracellular polyamines. The least-squares estimates of K 1/2 for putrescine, spermidine, and spermine were 3.0 mM, 80 μM, and 6.7 μM, respectively. These values are similar to those for extracellular polyamines at −40 mV (compare Figs. 3 and 6).
Figure 6.
Comparative potency of the three intracellular polyamines. The fraction of unblocked currents at +40 mV is plotted against the concentrations of putrescine, spermidine, and spermine. The solid curves are least-squares fits of equations of the forms I/Io = PA K 1/2/(PA K 1/2 + [PA]); see Fig. 3. The K 1/2 values for putrescine, spermidine, and spermine are 3.0 mM, 80 μM, and 6.7 μM, respectively.
discussion
In the present study, we discovered that both intracellular and extracellular polyamines block the CNG channel in a voltage-dependent manner. Of the three polyamines tested, spermine, with the greatest number of ammonium and methylene groups, blocked the channel with the strongest voltage dependence and the highest affinity. The value of K 1/2 was 7 μM for intracellular spermine at +40 mV and 3 μM for extracellular spermine at −40 mV. Because the higher affinity and stronger voltage dependence of spermine allow a more complete observation of its blocking behaviors within manageable ranges of concentration and membrane voltage, we will mainly discuss channel blockade by spermine.
As in some glutamate-gated channels (e.g., Bahring et al., 1997), extracellular spermine in the CNG channel acts like a typical permeant cationic pore blocker: reductions in positive membrane voltage enhances CNG channel blockade by spermine, whereas negative voltage relieved blockade (Figs. 1 and 2). Permeation of spermine through the CNG channel is physically possible because the estimated diameter of the CNG pore (∼6 Å) exceeds that of spermine (∼5 Å) (Goulding et al., 1993; Bahring et al., 1997). Compared with divalent cations, spermine (with up to four positive charges) appears to be more permeant over the same voltage range (e.g., compare Park and MacKinnon, 1995, with the present study).
CNG channel blockade by intracellular spermine is more complex. Unlike extracellular spermine, intracellular spermine does not simply behave as a permeant blocker. Nor does it act like a nonpermeant blocker as in inward-rectifier K+ channels (Ficker et al., 1994; Lopatin et al., 1994; Fakler et al., 1995). As suggested in Fig. 7, the complex behavior of spermine can be explained by assuming that spermine adopts two conformations, one permeant and the other non- (or much less) permeant. For example, spermine may act as a permeant blocker when it binds in a linear conformation along the narrow region of the pore. It may also act as a nonpermeant blocker when it adopts a “curled” conformation(s) and jams itself in the wider internal region of the pore, similar to blockade of the sarcoplasmic reticulum K+ channel by bis-quaternary ammonium compounds (Miller, 1982). The two conformations of spermine may be interconverted.
Figure 7.
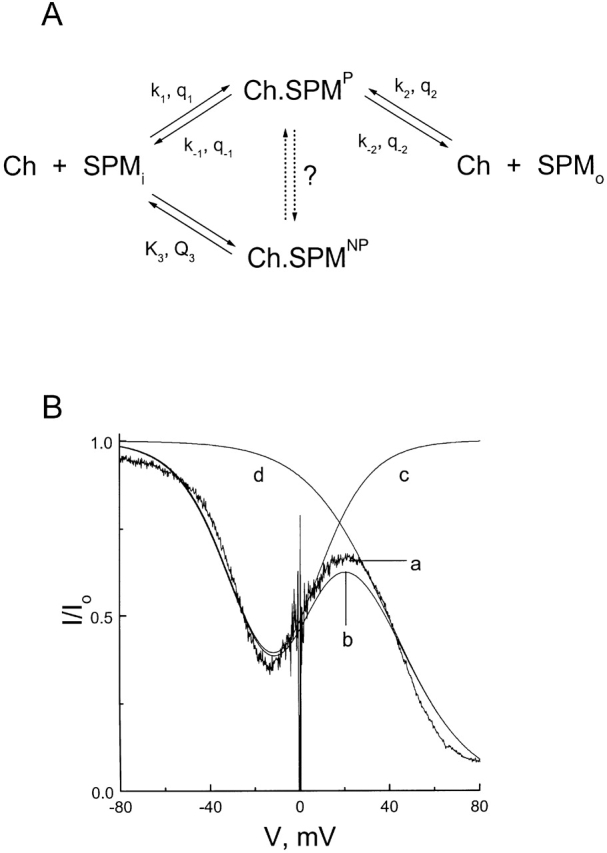
A kinetic model for the action of spermine. (A) Reaction scheme. Ch represents the CNG channel, SPMi and SPMo denote intra- and extracellular spermine, and Ch.SPMP and Ch.SPMNP denote the CNG channels blocked by spermine in the permeant and nonpermeant conformations, respectively. Kx and Qx are, respectively, the equilibrium dissociation constant at 0 mV and the total number of equivalent charges moving across the electrical field (in either direction) for nonpermeant spermine binding. Rate constants at 0 mV (kx or k−x) and the number of equivalent charges traversing the electrical field (qx or q−x) for movements of the permeant conformation of spermine are also shown. (The number of equivalent charges is defined as the actual number of charges of the blocker multiplied by the fraction of the electrical field transversed by the blocker.) (B) Noisy trace a is the same as that in Fig. 4 B, obtained in the presence of 10 μM of intracellular spermine. Curve b was drawn according to the scheme in A, with k1 = 1 * 106 M−1 s−1, k−1 = 8 s−1, k2 = 1.3 * 106 M−1 s−1, k−2 = 10.4 s−1, K3 = 333 μM, q1 = 2, q−1 = 0, q2 = 2, q−2 = 0, Q 3 = 1.5. (Any set of four rate constants in the given proportion will yield the same result.) Intracellular and extracellular spermine concentrations were set at 10 μM and zero, respectively. Curves c and d were drawn using the same parameters, except that both k1 and k−1 were zero for curve c and that K3 was infinite for curve d.
The noisy trace a in Fig. 7 B is from Fig. 4 B and shows how the fraction of unblocked current in the presence of 10 μM intracellular spermine varies with membrane voltage. Fig. 7 B, b corresponds to the model in Fig. 7 A (see below and the figure legend for details), which proposes that intracellular spermine can bind to the channel in either a permeant (e.g., linear) or a nonpermeant (e.g., curled) conformation (external spermine concentration was set at zero). Fig. 7 B, c and d, were drawn by assuming that spermine acts only as either a permeant or a nonpermeant blocker. Our model interprets the first and second descending phases as reflecting blockade by spermine in the permeant and nonpermeant conformations, respectively. The intervening ascending phase reflects spermine permeation. According to the model, we only observe such an interesting and complex blocking behavior when the pore has much higher affinity for the permeant than nonpermeant conformation of spermine. Fig. 7 B, b was drawn assuming that the pore has an ∼40-fold higher affinity for the permeant than nonpermeant conformation of spermine.
Fig. 7 A will also explain the action of extracellular spermine, provided one assumes that the binding state of the permeant form of spermine is the same regardless of the direction from which spermine enters the pore. In Fig. 8, we replotted the data from Figs. 2 F and 5 F. The theoretical curves superimposed on the data correspond to the model of Fig. 7 A, which allows extracellular spermine to act only as a permeant blocker, whereas intracellular spermine acts as a permeant as well as nonpermeant blocker. The model assumes that the binding of the permeant form of spermine is voltage independent, whereas the release of the permeant form of spermine into either the intracellular or the extracellular solution is voltage dependent. In the model, the spermine-binding site is located halfway through the transmembrane electrical field. (Although we have not exhausted all possibilities, the model appears to describe the data better if the voltage dependence mainly associates with the unbinding rather than the binding of spermine in the permeant form. The physical mechanism underlying this peculiar voltage dependence remains to be established.) Fig. 8 shows that this simple model qualitatively describes channel blockade by various concentrations of intracellular and extracellular spermine.
Figure 8.
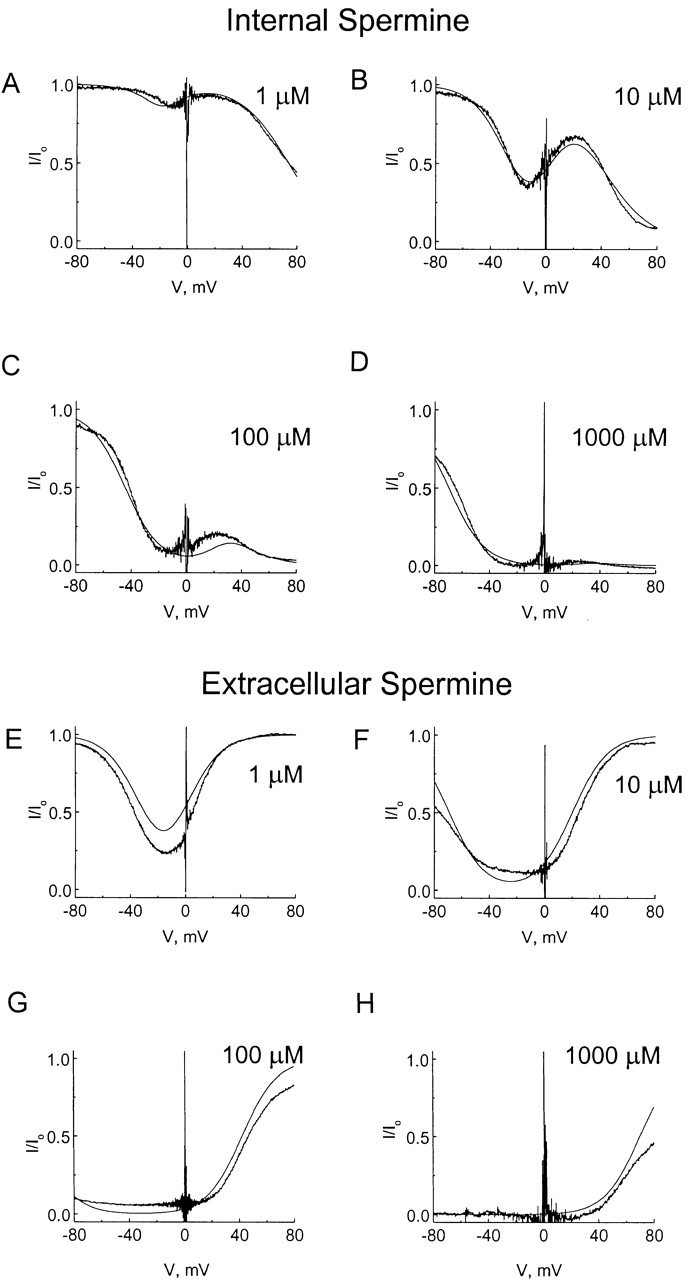
Modeling of channel blockade by various concentrations of intracellular and extracellular spermine. The experimental data (noisy traces) are the same as those in Figs. 2 F and 5 F. All smooth traces were computed according to the scheme in Fig. 7 A, using the same parameters as in Fig. 7 B and appropriate concentrations of intracellular and extracellular spermine. For example, in Fig. 8 A the model's intracellular spermine concentration was set at 1 μM and extracellular spermine concentration at zero.
Many ion channels, such as inward-rectifier K+ channels and some glutamate channels, are blocked by cations. The block of these channels is a critical feature that enables the channels to accomplish many important biological tasks. Initially, the block was attributed only to divalent cations (Ault et al., 1980; Mayer et al., 1984; Nowak et al., 1984; Horie et al., 1987; Matsuda et al., 1987; Vandenberg, 1987). Recently, polycationic polyamines were discovered to block inward-rectifier K+ channels and some glutamate-gated channels (Rock and Macdonald, 1992a,b; Araneda et al., 1993; Benveniste and Mayer, 1993; Lopatin et al., 1994; Ficker et al., 1994; Bowie and Mayer, 1995; Donevan and Rogawski, 1995; Fakler et al., 1995; Igarashi and Williams, 1995; Isa et al., 1995; Kamboj et al., 1995; Koh et al., 1995; Bahring, 1997; Williams, 1997). We have shown here that polyamines also block the CNG channel. Spermine blocks the inward-rectifier K+ channels as a nonpermeant blocker, whereas it blocks the glutamate-gated channels as a permeant blocker. In the CNG channel, spermine blocks the pore from the outside as a permeant blocker, qualitatively similar to that in the glutamate-gated channels. However, spermine blocks the CNG channel from the inside as a permeant as well as nonpermeant blocker, a combined behavior of spermine in the glutamate-gated channels and inward-rectifier K+ channels. The emerging pattern is that channels blocked by (or conducting) divalent cations can also be blocked by polyamines. Polyamines appear to block selective (e.g., inward-rectifier) cation channels in a nonpermeant manner, but nonselective (e.g., glutamate- and cyclic nucleotide–gated) channels in a permeant manner.
The physiological significance of CNG channel blockade by polyamines remains to be determined. Polyamines are ubiquitous in cells. The concentrations at which intracellular polyamines block the CNG channel are well within the concentration range for polyamines found in many cell types (e.g., Bahring et al., 1997). Polyamines also exist in extracellular fluid (e.g., Williams, 1997). However, most studies on CNG channel conduction were carried out with defined artificial extracellular solutions free of polyamines. As shown here, 1 μM extracellular spermine dramatically inhibits the inward Na+ current. This concentration is 100–1,000-fold lower than that required to block _N_-methyl-d-arginine–gated channels (Rock and Macdonald, 1992a,b; Araneda et al., 1993; Benveniste and Mayer, 1995a,b; Igarashi and Williams, 1995; Chao et al., 1997). It will be important to establish to what extent CNG channels are affected by extracellular polyamines in their native environment. Polyamines likely block permeation not only of monovalent cations but also of divalent cations such as Ca2+. Block of Ca2+ influx could have a major impact on the adaptation of visual transduction in vertebrate photoreceptors.
Acknowledgments
We thank S. Siegelbaum for the CNG channel cDNA, C.M. Armstrong, P. De Weer, A. Klem, and R. MacKinnon for their critical review of the manuscript.
This study was supported by a National Institutes of Health grant (GM-55560) and a grant from the Research Foundation of the University of Pennsylvania. Z. Lu was a recipient of the Independent Scientist Award from the NIH (K02-HL03814).
Abbreviations used in this paper
CNG
cyclic nucleotide–gated
I–V
current–voltage
references
- Araneda RC, Zukin RS, Bennett MVL. Effects of polyamines on NMDA-induced currents in rat hippocampal neurons: a whole-cell and single channel study. Neurosci Lett. 1993;152:107–112. doi: 10.1016/0304-3940(93)90495-7. [DOI] [PubMed] [Google Scholar]
- Ault B, Evans RH, Francis AA, Oakes DJ, Witkins JC. Selective depression of excitatory amino acid induced depolarization by magnesium ions in isolated spinal cord preparations. J Physiol. 1980;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader CR, Macleish PR, Schwartz EA. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol (Camb) 1979;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahring R, Boeie D, Benveniste M, Mayer ML. Permeation and block of the rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol (Camb) 1997;502:575–589. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian BL, Fein GL. The effects of sodium replacement on the responses of toad rads. J Physiol (Camb) 1982;330:331–347. doi: 10.1113/jphysiol.1982.sp014344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ. Electrical properties of the light-sensitive conductance of salamander rods. J Physiol (Camb) 1986;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M, Mayer ML. Multiple effects of spermine on N-methyl-d-aspartic acid receptor responses of rat cultured hippocampal neurons. J Physiol (Camb) 1993;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bododia RD, Detwiler PB. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol (Camb) 1985;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Capovilla M, Caretta A, Cervetto L, Torre V. Ionic movements through light-sensitive channels of toad rods. J Physiol (Camb) 1983;343:295–310. doi: 10.1113/jphysiol.1983.sp014893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Seiler N, Renault J, Kashiwagi K, Masuko T, Igarashi K, Williams K. N 1-Dansyl-spermine and N 1-(n-octanesulfonyl)-spermine, novel glutamate receptor antagonists: block and permeation of N-methyl-d-aspartate receptors. Mol Pharmacol. 1997;51:861–871. doi: 10.1124/mol.51.5.861. [DOI] [PubMed] [Google Scholar]
- Colamartino G, Menini A, Torre V. Blockade and permeation of divalent cations through the cyclic GMP-activated channel from tiger salamander retinal rods. J Physiol (Camb) 1991;440:189–206. doi: 10.1113/jphysiol.1991.sp018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. Intracellular polyamines mediate inward-rectification of Ca2+-permeable-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors. Proc Natl Acad Sci USA. 1995;92:9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eismann E, Muller F, Heinemann SH, Kaupp UB. A single negative charge within the pore controls rectification, Ca2+blockage, and ion selectivity. Proc Natl Acad Sci USA. 1994;91:1109–1113. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Branle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersburg JP. Strong voltage-dependent inward-rectification of inward-rectifier K+channels is caused by intracellular spermine. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Goulding EH, Ngai J, Kramer RH, Colicos S, Axel R, Siegelbaum SA, Chess A. Molecular cloning and single channel properties of the cyclic nucleotide-gated channel from catfish olfactory neurons. Neuron. 1992;8:45–58. doi: 10.1016/0896-6273(92)90107-o. [DOI] [PubMed] [Google Scholar]
- Goulding EH, Tibbs GR, Liu D, Siegelbaum SA. Role of H5 domain in determining pore diameter and ion permeation through cyclic nucleotide-gated channels. Nature. 1993;364:61–64. doi: 10.1038/364061a0. [DOI] [PubMed] [Google Scholar]
- Haynes LW, Kay AR, Yau K-W. Single cyclic GMP- activated channel activity in excised patches of rod outer segment membrane. Nature. 1986;321:66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ, Yau K-W. Effects of ions on retinal rods from Bufo marinus. . J Physiol (Camb) 1984;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ. The ion selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol (Camb) 1985;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M, Irisawa H, Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol (Camb) 1987;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Williams K. Antagonist properties of polyamines and bis(ethyl)polyamines at N-methyl-d-aspartate receptors. J Pharmacol Exp Ther. 1995;272:1101–1109. [PubMed] [Google Scholar]
- Isa T, Iino M, Itazawa S, Ozawa S. Spermine mediates inward-rectification of Ca2+permeable AMPA receptor channels. Neuroreport. 1995;6:2045–2048. doi: 10.1097/00001756-199510010-00022. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium permeable AMPA and kainate receptors. J Physiol (Camb) 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB, Koch K-W. Role of cGMP and Ca2+in vertebrate photoreceptor excitation and adaptation. Annu Rev Physiol. 1992;54:153–175. doi: 10.1146/annurev.ph.54.030192.001101. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Niidome T, Tanabe T, Terada S, Baningk W, Stühmer W, Cook N, Kangawa K, Matsuo H, Hirose T, Numa S. Primary structure and functional expression from complementary DNA of rod photoreceptor cyclic GMP-gated channel. Nature. 1989;342:283–292. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Koh DS, Burnashey N, Jonas P. Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol (Camb) 1995;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalous Y, Yau K-W. A rich complexity emerges in phototransduction. Curr Opin Neurobiol. 1993;3:513–519. doi: 10.1016/0959-4388(93)90049-5. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhaaina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inward-rectifier K+ channel and blocking by internal Mg2+ . Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage- dependent block by Mg2+of NMDA responses in spinal cord neurons. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Miller C. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+channel. J Gen Physiol. 1982;79:869–891. doi: 10.1085/jgp.79.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Yau K-W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol (Camb) 1988;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Park C-S, MacKinnon R. Divalent cation selectivity in a cyclic nucleotide-gated ion channel. Biochemistry. 1995;34:13326–13333. doi: 10.1021/bi00041a008. [DOI] [PubMed] [Google Scholar]
- Rock DM, Macdonald RL. The polyamine spermine has multiple actions on N-methyl-d-aspartate receptor single-channel currents in cultured cortical neurons. Mol Pharmacol. 1992a;41:83–88. [PubMed] [Google Scholar]
- Rock DM, Macdonald RL. Spermine and related polyamines produce a voltage-dependent reduction of N-methyl- d-aspartate receptor single-channel conductance. Mol Pharmacol. 1992b;42:157–164. [PubMed] [Google Scholar]
- Root MJ, MacKinnon R. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron. 1993;11:459–466. doi: 10.1016/0896-6273(93)90150-p. [DOI] [PubMed] [Google Scholar]
- Stern JH, Knutsson H, Macleish PR. Divalent cations directly affect the conductance of excised patches of rod photoreceptor membrane. Science. 1987;236:1674–1678. doi: 10.1126/science.3037695. [DOI] [PubMed] [Google Scholar]
- Torre V, Matthews HR, Lamb TD. Ion selectivity, blockage and control of light-sensitive conductance. Neurosci Res. 1987;6:S25–S44. doi: 10.1016/0921-8696(87)90005-3. [DOI] [PubMed] [Google Scholar]
- Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci USA. 1987;84:2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Regeneration of hyperpolarization in rods. J Physiol (Camb) 1975;244:53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell Signal. 1997;9:1–13. doi: 10.1016/s0898-6568(96)00089-7. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Fain GL, Bastian BL. Light-dependent ion flux into toad photoreceptors. J Gen Physiol. 1982;80:517–536. doi: 10.1085/jgp.80.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K-W, Baylor DA. Cyclic CMP-activated conductance of retinal photoreceptor. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K-W, McNaughton PA, Hodgkin AL. Effects of ions on the light-sensitive current in retinal rods. Nature. 1981;292:502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yau K-W, Nakatini K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984;309:352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman AL, Baylor DA. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J Physiol (Camb) 1992;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]