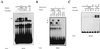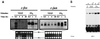Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression - PubMed (original) (raw)
Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression
A L Armesilla et al. Mol Cell Biol. 1999 Mar.
Abstract
Vascular endothelial growth factor (VEGF) is a potent angiogenic inducer that stimulates the expression of tissue factor (TF), the major cellular initiator of blood coagulation. Here we show that signaling triggered by VEGF induced DNA-binding and transcriptional activities of nuclear factor of activated T cells (NFAT) and AP-1 in human umbilical vein endothelial cells (HUVECs). VEGF also induced TF mRNA expression and gene promoter activation by a cyclosporin A (CsA)-sensitive mechanism. As in lymphoid cells, NFAT was dephosphorylated and translocated to the nucleus upon activation of HUVECs, and these processes were blocked by CsA. NFAT was involved in the VEGF-mediated TF promoter activation as evidenced by cotransfection experiments with a dominant negative version of NFAT and site-directed mutagenesis of a newly identified NFAT site within the TF promoter that overlaps with a previously identified kappaB-like site. Strikingly, this site bound exclusively NFAT not only from nuclear extracts of HUVECs activated by VEGF, a stimulus that failed to induce NF-kappaB-binding activity, but also from extracts of cells activated with phorbol esters and calcium ionophore, a combination of stimuli that triggered the simultaneous activation of NFAT and NF-kappaB. These results implicate NFAT in the regulation of endothelial genes by physiological means and shed light on the mechanisms that switch on the gene expression program induced by VEGF and those regulating TF gene expression.
Figures
FIG. 1
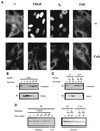
VEGF induces dephosphorylation and nuclear localization of NFATp in HUVECs. (A) Immunocytochemical staining of HUVECs unstimulated (−) or treated with VEGF (50 ng/ml), the calcium ionophore A23187 (1 μM) (I0), or 25 ng of TNF-α per ml for 20 min. The cells were either untreated (top panels) or incubated with 200 ng of CsA per ml (bottom panels) for 2 h prior to the addition of the stimuli. After stimulation, the cells were fixed and stained with the anti-NFATp antiserum 67.1. (B and C) HUVECs, either pretreated or not (−) with CsA (200 ng/ml) for 2 h, were left untreated (−) or stimulated with VEGF (50 ng/ml) or calcium ionophore (I0) (1 μM) for the indicated times. Fractionated cytoplasmic or nuclear extracts were analyzed by Western blotting with the 67.1 antiserum for NFATp detection. The mobilities of the upper and lower bands, corresponding to phosphorylated and dephosphorylated forms of NFATp, respectively, are indicated by arrows. (D) As a control for the fractionation process, aliquots of the extracts used for NFATp detection were analyzed by Western blotting for the presence of nuclear PCNA. Cyt, cytoplasmic; V, VEGF.
FIG. 2
Kinetic analysis and serological characterization of the NFAT DNA-binding complexes induced by VEGF. Nuclear extracts from HUVECs stimulated for the indicated times with VEGF (50 ng/ml) (V) or a combination of PMA (20 ng/ml) plus ionophore (1 μM) (P/I0) or pretreated with CsA (200 ng/ml) for 2 h before stimulation were analyzed by EMSA. (A) Analysis of the DNA-binding activity to the NFAT probe of the IL-4 promoter in nuclear extracts from HUVECs activated for different times with VEGF (VEGF or V). EMSAs with extracts from VEGF-activated cells pretreated with CsA as well as control extracts from PMA- plus ionophore-treated cells are shown. The mobility of the specific VEGF-induced (CsA-sensitive) complex is indicated by an arrow. (B) Serological characterization of the NFAT DNA-binding complexes. EMSAs were performed in the presence (+) or absence (−) of nuclear extracts from cells activated with VEGF or PMA plus ionophore that were incubated with 0.5 μl of either preimmune serum (Preim.), anti-NFATp antiserum 67.1, or the anti-NFAT family antiserum 796 for 15 min prior to the addition of the labeled probe. The VEGF-induced NFAT complex and the supershifted complexes induced by the anti-NFATp 67.1 are indicated by an arrow and asterisks, respectively. (C) Nuclear extracts from HUVECs treated as in panel A were analyzed for NF-κB binding with the κB site of the IL-2 promoter as a probe.
FIG. 3
Effects of VEGF on the mRNA levels of c-fos and c-jun and on the DNA-binding activity of AP-1. (A) Northern blot analysis with total RNA from HUVECs untreated or activated for the indicated times with VEGF or a combination of PMA plus ionophore (P/I0). After isolation, RNA was separated by agarose gel electrophoresis, blotted onto a nitrocellulose membrane, and hybridized with specific probes for c-fos and c-jun. RNA controls of the corresponding blots are shown. A sixfold-longer exposure of the autoradiograph is presented for the control and VEGF points of c-fos. (B) The AP-1 DNA-binding activity displayed by nuclear extracts from HUVECs activated with VEGF or PMA plus ionophore (P/I0) for 1 h was tested with a specific probe encompassing the −284 AP-1 site of the ICAM-1 promoter. Extracts from CsA-treated cells were obtained after pretreatment of 2 h with 200 ng of CsA per ml and a further 1-h treatment with the stimuli. VEGF (50 ng/ml), PMA (20 ng/ml), and A23187 calcium ionophore (1 μM) were used at the same doses in single or combined treatments in panels A and B.
FIG. 4
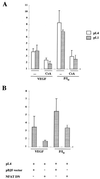
VEGF activates AP-1- and NFAT-dependent transcription- (A) HUVECs were transfected by the calcium phosphate procedure with the AP-1-responsive (−73 to +63) region of the collagenase promoter plasmid for 4.5 h and stimulated or left untreated for an additional 16 h, and then the luciferase activity was determined. The data is expressed as the mean and standard deviation (error bars) of determinations performed in triplicate. Results of a representative experiment are shown. Four different experiments yielded similar results. (B) HUVECs were transfected with an NFAT-dependent luciferase reporter plasmid. At 16 h after transfection, the cells were pretreated or not (−) with CsA (200 ng/ml) for 2 h, and then left untreated or further stimulated for an additional 6 h with VEGF or a combination of PMA and calcium ionophore (P/I0). The results are expressed as the relative fold induction over the relative luciferase units (RLU) displayed by the corresponding transfected unstimulated cells. Results of a representative experiment of five are shown. VEGF (50 ng/ml), PMA (20 ng/ml), and A23187 calcium ionophore (1 μM) were used at the same doses in single or combined treatments in panels A and B.
FIG. 5
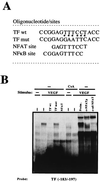
NFATp binds to the −183 to −197 region within the TF promoter. (A) The sequences (5′ to 3′) of the oligonucleotides including the −183 to −197 region of the TF promoter (TF wt) and that of the TF mut are shown. Base pair substitutions incorporated in the TF mut are indicated by asterisks. The partially overlapping nucleotides corresponding to the κB-like and the NFAT sites are indicated. (B) Nuclear extracts from HUVECs pretreated or not with 200 ng of CsA per ml and then exposed to VEGF (50 ng/ml) were analyzed by EMSA with a probe spanning positions −183 to −197 of the TF promoter. A 30-fold molar excess of unlabeled TF −183 to −197, the corresponding oligonucleotide mutated at positions −186 to −194 (TF mut), or an NFAT consensus site of the IL-4 promoter oligonucleotides was added to the binding-reaction mixtures to detect the specific binding. Serological identification of the complexes was performed by addition of 0.5 μl of either preimmune serum (Preim.), anti-NFATp antiserum (67.1), or anti-NFAT family antiserum (796). Antisera and cold oligonucleotides were added to the binding-reaction mixture prior to addition of the probe.
FIG. 6
Serological analysis of the binding to the TF/NFAT site induced by VEGF and PMA plus ionophore. The DNA binding to the −183 to −197 region of the TF promoter was analyzed by using nuclear extracts from HUVECs treated with VEGF (50 ng/ml) or with PMA (20 ng/ml) plus A23187 calcium ionophore (1 μM) for 1 h. Anti-NFATp antiserum 67.1 (0.5 μl) or 1 μl of anti-p65 antisera was added to the binding-reaction mixture before the addition of the probe.
FIG. 7
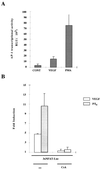
Effects of CsA and NFAT dominant negative plasmid on the transcription of the TF promoter by VEGF. (A) The transcriptional activity of the TF promoter was tested by transfection of HUVECs. The pL4 and pL1 luciferase reporter plasmids (10 μg) were transfected by calcium phosphate precipitation for 4.5 h. At 16 h posttransfection, cells were preincubated or not (−) with CsA for 2 h, and further stimulated for 6 h with VEGF, PMA plus ionophore (P/I0), or vehicle. Results of a representative experiment of three independent experiments performed are shown. (B) The pL4 promoter construct (5 μg) was cotransfected with 5 μg of either the NFAT dominant negative pSH102CΔ418 expression vector or its parental empty vector (pBJ5). At 16 h after transfection, the cells were stimulated with VEGF, PMA plus ionophore (P/I0), or vehicle for 6 h. Results of a representative experiment of three independent ones are presented. The results are expressed as fold induction over the expression of pL4 and pL1 plasmids in the absence of stimuli in both panels. Experiments were performed in triplicate. VEGF (50 ng/ml), PMA (20 ng/ml), CsA (200 ng/ml), and A23187 calcium ionophore (1 μM) were used at the same doses in single and combined treatments.
FIG. 8
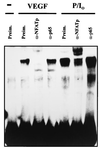
Inhibition of VEGF-induced TF expression by CsA. (A) HUVECs were transfected by calcium phosphate precipitation with 10 μg of the parental pL4 luciferase plasmid or with the pL4mut-derived plasmid mutated in the NFAT site for 4.5 h. At 16 h posttransfection, the cells were stimulated for 6 h with VEGF (50 ng/ml) or PMA (20 ng/ml) plus ionophore (1 μM). Experiments were performed in triplicate, and the results are expressed as fold induction over the baseline levels of transfected unstimulated cells. Results of a representative experiment of three performed are shown. (B) Northern blot analysis of HUVECs pretreated or not (−) with CsA (200 ng/ml) for 2 h and further stimulated with VEGF (50 ng/ml) or a combination of PMA (20 ng/ml) plus ionophore (1 μM) (P/I0) for the time points indicated. Total RNA was electrophoresed, blotted onto a nitrocellulose membrane, and hybridized with a TF cDNA probe. Autoradiographs corresponding to exposures for VEGF and PMA-plus-ionophore treatments of 1 week and 16 h, respectively, and RNA controls of the blot are presented.
Similar articles
- Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2.
Hernández GL, Volpert OV, Iñiguez MA, Lorenzo E, Martínez-Martínez S, Grau R, Fresno M, Redondo JM. Hernández GL, et al. J Exp Med. 2001 Mar 5;193(5):607-20. doi: 10.1084/jem.193.5.607. J Exp Med. 2001. PMID: 11238591 Free PMC article. - Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT.
Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, Hofer E, Binder BR, Leitinger N. Bochkov VN, et al. Blood. 2002 Jan 1;99(1):199-206. doi: 10.1182/blood.v99.1.199. Blood. 2002. PMID: 11756172 - Specificity, diversity, and convergence in VEGF and TNF-alpha signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells.
Mechtcheriakova D, Schabbauer G, Lucerna M, Clauss M, De Martin R, Binder BR, Hofer E. Mechtcheriakova D, et al. FASEB J. 2001 Jan;15(1):230-242. doi: 10.1096/fj.00-0247com. FASEB J. 2001. PMID: 11149911 - Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family.
Zachary I, Gliki G. Zachary I, et al. Cardiovasc Res. 2001 Feb 16;49(3):568-81. doi: 10.1016/s0008-6363(00)00268-6. Cardiovasc Res. 2001. PMID: 11166270 Review. - Signalling into the T-cell nucleus: NFAT regulation.
Masuda ES, Imamura R, Amasaki Y, Arai K, Arai N. Masuda ES, et al. Cell Signal. 1998 Oct;10(9):599-611. doi: 10.1016/s0898-6568(98)00019-9. Cell Signal. 1998. PMID: 9794241 Review.
Cited by
- Loss of Down Syndrome Critical Region-1 Mediated-Hypercholesterolemia Accelerates Corneal Opacity Via Pathological Neovessel Formation.
Muramatsu M, Nakagawa S, Osawa T, Toyono T, Uemura A, Kidoya H, Takakura N, Usui T, Ryeom S, Minami T. Muramatsu M, et al. Arterioscler Thromb Vasc Biol. 2020 Oct;40(10):2425-2439. doi: 10.1161/ATVBAHA.120.315003. Epub 2020 Aug 13. Arterioscler Thromb Vasc Biol. 2020. PMID: 32787520 Free PMC article. - Lessons from dermatology about inflammatory responses in Covid-19.
Criado PR, Pagliari C, Carneiro FRO, Quaresma JAS. Criado PR, et al. Rev Med Virol. 2020 Sep;30(5):e2130. doi: 10.1002/rmv.2130. Epub 2020 Jul 12. Rev Med Virol. 2020. PMID: 32656939 Free PMC article. Review. - Angiogenesis, thrombogenesis, endothelial dysfunction and angiographic severity of coronary artery disease.
Chung NA, Lydakis C, Belgore F, Li-Saw-Hee FL, Blann AD, Lip GY. Chung NA, et al. Heart. 2003 Dec;89(12):1411-5. doi: 10.1136/heart.89.12.1411. Heart. 2003. PMID: 14617549 Free PMC article. - Epithelial calcineurin controls microbiota-dependent intestinal tumor development.
Peuker K, Muff S, Wang J, Künzel S, Bosse E, Zeissig Y, Luzzi G, Basic M, Strigli A, Ulbricht A, Kaser A, Arlt A, Chavakis T, van den Brink GR, Schafmayer C, Egberts JH, Becker T, Bianchi ME, Bleich A, Röcken C, Hampe J, Schreiber S, Baines JF, Blumberg RS, Zeissig S. Peuker K, et al. Nat Med. 2016 May;22(5):506-15. doi: 10.1038/nm.4072. Epub 2016 Apr 4. Nat Med. 2016. PMID: 27043494 Free PMC article. - C/EBPβ and Nuclear Factor of Activated T Cells Differentially Regulate Adamts-1 Induction by Stimuli Associated with Vascular Remodeling.
Oller J, Alfranca A, Méndez-Barbero N, Villahoz S, Lozano-Vidal N, Martín-Alonso M, Arroyo AG, Escolano A, Armesilla AL, Campanero MR, Redondo JM. Oller J, et al. Mol Cell Biol. 2015 Oct;35(19):3409-22. doi: 10.1128/MCB.00494-15. Epub 2015 Jul 27. Mol Cell Biol. 2015. PMID: 26217013 Free PMC article.
References
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. - PubMed
- Andersen H O, Madsen G, Nordestgaard B G, Hansen B F, Kjeldsen K, Stender S. Cyclosporin suppresses transplant arteriosclerosis in the aorta-allografted, cholesterol-clamped rabbit. Suppression preceded by decrease in arterial lipoprotein permeability. Arterioscler Thromb. 1994;14:944–950. - PubMed
- Brown L F, Detmar M, Claffey K, Nagy J A, Feng D, Dvorak A M, Dvorak H F. Vascular permeabilty factor/vascular endothelial growth factor: A multifunctional angiogenic cytokine. In: Rosen G A, editor. Regulation of angiogenesis. Basel, Switzerland: Birkhauser Verlag; 1997. pp. 233–269. - PubMed
- Cahill M A, Janknecht R, Nordheim A. Signalling pathways: jack of all cascades. Curr Biol. 1996;6:16–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous