Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae - PubMed (original) (raw)
Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae
D A Schuhmacher et al. J Bacteriol. 1999 Mar.
Abstract
The regulatory protein ToxT directly activates the transcription of virulence factors in Vibrio cholerae, including cholera toxin (CT) and the toxin-coregulated pilus (TCP). Specific environmental signals stimulate virulence factor expression by inducing the transcription of toxT. We demonstrate that transcriptional activation by the ToxT protein is also modulated by environmental signals. ToxT expressed from an inducible promoter activated high-level expression of CT and TCP in V. cholerae at 30 degrees C, but expression of CT and TCP was significantly decreased or abolished by the addition of 0.4% bile to the medium and/or an increase of the temperature to 37 degrees C. Also, expression of six ToxT-dependent TnphoA fusions was modulated by temperature and bile. Measurement of ToxT-dependent transcription of genes encoding CT and TCP by ctxAp- and tcpAp-luciferase fusions confirmed that negative regulation by 37 degrees C or bile occurs at the transcriptional level in V. cholerae. Interestingly, ToxT-dependent transcription of these same promoters in Salmonella typhimurium was relatively insensitive to regulation by temperature or bile. These data are consistent with ToxT transcriptional activity being modulated by environmental signals in V. cholerae and demonstrate an additional level of complexity governing the expression of virulence factors in this pathogen. We propose that negative regulation of ToxT-dependent transcription by environmental signals prevents the incorrect temporal and spatial expression of virulence factors during cholera pathogenesis.
Figures
FIG. 1
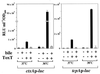
ToxT-dependent expression of CT and TCP is modulated by temperature and bile in a V. cholerae wild-type strain. V. cholerae O395 carrying plasmid pKEK162, which expresses ToxT from the P_lac_ promoter, was grown as described in Materials and Methods in LB containing 0.3 mM IPTG in the absence (−) or presence (+) of 0.4% bile at 30 or 37°C. Arrows indicate agglutinated cells resulting from TCP expression. CT levels in culture supernatants were determined as described in Materials and Methods.
FIG. 2
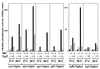
ToxT-dependent Tn_phoA_ fusions are modulated by temperature and bile in the absence of ToxR. V. cholerae strains containing a Δ_toxR1_ mutation and Tn_phoA_ fusions to ToxT-dependent genes and carrying either plasmid pKEK162, which expresses ToxT from the P_lac_ promoter (ToxT +), or the vector pUC118 (ToxT −) were grown as described in Materials and Methods in LB containing 0.3 mM IPTG in the absence (black bars) or presence (hatched bars) of 0.4% bile at 30 or 37°C. The V. cholerae strains used were KKV356 (ctx::Tn_phoA_), KK357 (acfA::Tn_phoA_), KKV358 (acfB::Tn_phoA_), KKV359 (acfC::Tn_phoA_), KKV362 (tcpI::Tn_phoA_), and KKV363 (tagA::Tn_phoA_). Alkaline phosphatase activities are the averages and standard deviations from three samples (note differences in scale).
FIG. 3
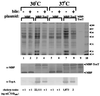
Expression of MBP-ToxT from P_lac_ is not affected by temperature or bile. V. cholerae KKV365 (Δ_toxR1_ Δ_toxT_) carrying either plasmid pKEK169, which expresses MBP-ToxT from the P_lac_ promoter (lanes 3, 4, 7, and 8), or pKEK168, which expresses MBP from the P_lac_ promoter (lanes 1, 2, 5, and 6), was grown as described in Materials and Methods in LB containing 0.3 mM IPTG in the absence (lanes 1, 3, 5, and 7) or presence (lanes 2, 4, 6, and 8) of 0.4% bile at 30°C (lanes 1 to 4) or 37°C (lanes 5 to 8). Whole-cell lysates were matched by OD600, separated on a sodium dodecyl sulfate–12% polyacrylamide gel, and then stained with Coomassie blue (upper panel). Lane 9, partially purified MBP-ToxT; lane 10, molecular weight standards (weights are in thousands). The samples in lanes 1 to 9 were subjected to Western analysis (see Materials and Methods) with rabbit polyclonal antiserum against MBP (α-MBP; middle panel); MBP-ToxT and MBP are indicated by arrowheads. The samples in lanes 1 to 8 were also subjected to Western analysis with rabbit polyclonal antiserum against TcpA (α-TcpA, bottom panel). CT in culture supernatants corresponding to the samples in lanes 1 to 8 was quantitated as described in Materials and Methods.
FIG. 4
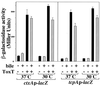
ToxT-dependent transcription of ctxA and tcpA is modulated by temperature and bile in V. cholerae. Δ_toxR1_ Δ_toxT V. cholerae_ strains containing chromosomal ctxAp-luc (KKV523) and tcpAp-luc (KKV515) transcriptional fusions and carrying either plasmid pKEK162, which expresses ToxT from the P_lac_ promoter (ToxT +), or the vector pUC118 (ToxT −) were grown as described in Materials and Methods in LB containing 0.3 mM IPTG in the absence (black bars) or presence (hatched bars) of 0.4% bile at 30 or 37°C. Cultures were assayed for luciferase activity as described in Materials and Methods. Results are the averages and standard deviations from three samples. RLU, relative light units.
FIG. 5
ToxT-dependent transcription of ctxA and tcpA is relatively insensitive to temperature and bile in S. typhimurium. S. typhimurium strains containing chromosomal ctxAp-lacZ (KK201) and tcpAp-lacZ (KK207) transcriptional fusions and carrying either plasmid pKEK162, which expresses ToxT from the P_lac_ promoter (ToxT +), or the vector pUC118 (ToxT −) were grown as described in Materials and Methods (with streptomycin omitted from the medium) in LB containing 0.3 mM IPTG in the absence (black bars) or presence (hatched bars) of 0.4% bile at 30 or 37°C. Cultures were assayed for β-galactosidase as described in Materials and Methods. Results are the averages and standard deviations from three samples.
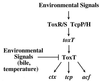
FIG. 6
Model of environmental regulation of the cascade controlling V. cholerae virulence. It has previously been shown that environmental signals modulate transcription of toxT by the ToxR-ToxS and TcpP-TcpH regulatory systems (3, 12); the present study demonstrates that environmental signals (temperature and bile) negatively regulate transcriptional activation of virulence factors (ctx, tcp, and acf) by the ToxT protein. Environmental regulation of V. cholerae virulence thus influences multiple levels of this regulatory cascade.
Similar articles
- Organization of tcp, acf, and toxT genes within a ToxT-dependent operon.
Brown RC, Taylor RK. Brown RC, et al. Mol Microbiol. 1995 May;16(3):425-39. doi: 10.1111/j.1365-2958.1995.tb02408.x. Mol Microbiol. 1995. PMID: 7565104 - pepA, a gene mediating pH regulation of virulence genes in Vibrio cholerae.
Behari J, Stagon L, Calderwood SB. Behari J, et al. J Bacteriol. 2001 Jan;183(1):178-88. doi: 10.1128/JB.183.1.178-188.2001. J Bacteriol. 2001. PMID: 11114915 Free PMC article. - Regulation of virulence in Vibrio cholerae.
Klose KE. Klose KE. Int J Med Microbiol. 2001 May;291(2):81-8. doi: 10.1078/1438-4221-00104. Int J Med Microbiol. 2001. PMID: 11437342 Review. - Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae.
Yu RR, DiRita VJ. Yu RR, et al. J Bacteriol. 1999 Apr;181(8):2584-92. doi: 10.1128/JB.181.8.2584-2592.1999. J Bacteriol. 1999. PMID: 10198025 Free PMC article. - The complexity of ToxT-dependent transcription in Vibrio cholerae.
Weber GG, Klose KE. Weber GG, et al. Indian J Med Res. 2011 Feb;133(2):201-6. Indian J Med Res. 2011. PMID: 21415495 Free PMC article. Review.
Cited by
- Survival of the Fittest: How Bacterial Pathogens Utilize Bile To Enhance Infection.
Sistrunk JR, Nickerson KP, Chanin RB, Rasko DA, Faherty CS. Sistrunk JR, et al. Clin Microbiol Rev. 2016 Oct;29(4):819-36. doi: 10.1128/CMR.00031-16. Clin Microbiol Rev. 2016. PMID: 27464994 Free PMC article. Review. - Bile salt receptor complex activates a pathogenic type III secretion system.
Li P, Rivera-Cancel G, Kinch LN, Salomon D, Tomchick DR, Grishin NV, Orth K. Li P, et al. Elife. 2016 Jul 5;5:e15718. doi: 10.7554/eLife.15718. Elife. 2016. PMID: 27377244 Free PMC article. - Reduced virulence of the Vibrio cholerae fadD mutant is due to induction of the extracytoplasmic stress response.
Chatterjee E, Chowdhury R. Chatterjee E, et al. Infect Immun. 2013 Oct;81(10):3935-41. doi: 10.1128/IAI.00722-13. Epub 2013 Aug 5. Infect Immun. 2013. PMID: 23918781 Free PMC article. - Cyclic diguanylate regulates Vibrio cholerae virulence gene expression.
Tischler AD, Camilli A. Tischler AD, et al. Infect Immun. 2005 Sep;73(9):5873-82. doi: 10.1128/IAI.73.9.5873-5882.2005. Infect Immun. 2005. PMID: 16113306 Free PMC article. - Regulatory networks controlling Vibrio cholerae virulence gene expression.
Matson JS, Withey JH, DiRita VJ. Matson JS, et al. Infect Immun. 2007 Dec;75(12):5542-9. doi: 10.1128/IAI.01094-07. Epub 2007 Sep 17. Infect Immun. 2007. PMID: 17875629 Free PMC article. Review. No abstract available.
References
- Brown R C, Taylor R K. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol Microbiol. 1995;16:425–439. - PubMed
- Champion G A, Neely M N, Brennan M A, DiRita V J. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources