Peripheral autoantigen induces regulatory T cells that prevent autoimmunity - PubMed (original) (raw)
Comparative Study
Peripheral autoantigen induces regulatory T cells that prevent autoimmunity
B Seddon et al. J Exp Med. 1999.
Abstract
Previous studies have shown that autoimmune thyroiditis can be induced in normal laboratory rats after thymectomy and split dose gamma-irradiation. Development of disease can be prevented by reconstitution of PVG rats shortly after their final irradiation with either peripheral CD4(+)CD45RC- T cells or CD4(+)CD8(-) thymocytes from syngeneic donors. Although the activity of both populations is known to depend on the activities of endogenously produced interleukin 4 and transforming growth factor beta, implying a common mechanism, the issue of antigen specificity of the cells involved has not yet been addressed. In this study, we show that the regulatory T cells that prevent autoimmune thyroiditis are generated in vivo only when the relevant autoantigen is also present. Peripheral CD4(+) T cells, from rats whose thyroids were ablated in utero by treatment with 131I, were unable to prevent disease development upon adoptive transfer into thymectomized and irradiated recipients. This regulatory deficit is specific for thyroid autoimmunity, since CD4(+) T cells from 131I-treated PVG.RT1(u) rats were as effective as those from normal donors at preventing diabetes in thymectomized and irradiated PVG.RT1(u) rats. Significantly, in contrast to the peripheral CD4(+) T cells, CD4(+)CD8(-) thymocytes from 131I-treated PVG donors were still able to prevent thyroiditis upon adoptive transfer. Taken together, these data indicate that it is the peripheral autoantigen itself that stimulates the generation of the appropriate regulatory cells from thymic emigrant precursors.
Figures
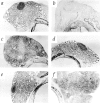
Figure 1
Morphology of thyroid glands in 131I-treated rats and TxX rats with thyroiditis. Pregnant PVG rats were injected intraperitoneally with 2 mCi of 131I on day 18 of gestation. Thyroid cartilage and attached tissue was removed from normal PVG rats (A) and 131I-treated rats (B) at 8 wk of age, sectioned and stained with hematoxylin and eosin. In the same series of experiments as those described in Fig. 2, whole thyroid glands attached to thyroid cartilage were taken at the time of peak disease from control TxX PVG rats (C), recipients of 107 CD4+ T cells from normal PVG rats (D), and recipients of either 5 × 106 CD4+CD8− thymocytes (E) or 107 CD4+ T cells (F) from the same athyroid donors. Thyroids were frozen, sectioned, and stained with hematoxylin and eosin and are shown at low magnification. (Original magnification: ×50.)
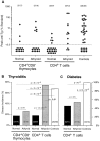
Figure 2
Adoptive transfer of peripheral CD4+ T cells from athyroid donors into TxX rats prevents development of diabetes but not thyroiditis. Athyroid donor PVG and PVG.RT1u rats were generated after exposure to 131I in utero as described in Fig. 1. Normal PVG and PVG.RT1u rats were thymectomized at 3 and 6 wk of age, respectively, and given 1,100 and 1,000 rads of split dose γ-irradiation, respectively, in four equal doses at 2-wk intervals. Shortly after the last irradiation, groups of TxX PVG rats were reconstituted with 5 × 106 CD4+CD8− thymocytes purified from the thymus of either normal or athyroid 8-wk-old PVG donor rats. Further groups of TxX PVG rats were reconstituted with 107 CD4+ T cells purified from the same normal and athyroid PVG donors. Development of anti-Tg IgG responses was monitored for between 4 and 12 wk after the last irradiation by specific ELISA. Data represent peak anti-Tg IgG titers of individual TxX rats, expressed as percentage of standard (A). TxX PVG.RT1u rats were reconstituted shortly after their final irradiation with 107 CD4+ T cells purified from either normal or athyroid 8-wk-old PVG.RT1u rats. The disease incidence of thyroiditis in groups of TxX PVG rats (B) and diabetes in groups of TxX PVG.RT1u rats (C) reconstituted with different cell subsets from different donors are expressed as a percentage of the group. Numbers in parentheses show the actual incidence and group sizes. P values were calculated using Fisher's exact test.
Similar articles
- Thymectomy and radiation-induced type 1 diabetes in nonlymphopenic BB rats.
Ramanathan S, Bihoreau MT, Paterson AD, Marandi L, Gauguier D, Poussier P. Ramanathan S, et al. Diabetes. 2002 Oct;51(10):2975-81. doi: 10.2337/diabetes.51.10.2975. Diabetes. 2002. PMID: 12351436 - Role of CD4+CD8- thymocytes in the prevention of autoimmune diabetes.
Seddon B, Mason D. Seddon B, et al. Biochem Soc Trans. 1997 May;25(2):620-4. doi: 10.1042/bst0250620. Biochem Soc Trans. 1997. PMID: 9191168 Review. No abstract available. - Autoreactive T cells in human type 1 diabetes.
Tree TI, Peakman M. Tree TI, et al. Endocrinol Metab Clin North Am. 2004 Mar;33(1):113-33, ix-x. doi: 10.1016/S0889-8529(03)00081-1. Endocrinol Metab Clin North Am. 2004. PMID: 15053898 Review. No abstract available.
Cited by
- Adoptive T Regulatory Cell Therapy for Tolerance Induction.
Cabello-Kindelan C, Mackey S, Bayer AL. Cabello-Kindelan C, et al. Curr Transplant Rep. 2015 Jun 1;2(2):191-201. doi: 10.1007/s40472-015-0058-5. Curr Transplant Rep. 2015. PMID: 25938011 Free PMC article. - Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system.
Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Kipnis J, et al. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15620-5. doi: 10.1073/pnas.232565399. Epub 2002 Nov 12. Proc Natl Acad Sci U S A. 2002. PMID: 12429857 Free PMC article. - Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node.
Samy ET, Parker LA, Sharp CP, Tung KS. Samy ET, et al. J Exp Med. 2005 Sep 19;202(6):771-81. doi: 10.1084/jem.20041033. J Exp Med. 2005. PMID: 16172257 Free PMC article. - Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis.
Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Kullberg MC, et al. J Exp Med. 2002 Aug 19;196(4):505-15. doi: 10.1084/jem.20020556. J Exp Med. 2002. PMID: 12186842 Free PMC article.
References
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. - PubMed
- Bonomo A, Kehn P, Shevach E. Post-thymectomy autoimmunity: abnormal T-cell homeostasis. Immunol Today. 1995;16:61–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials