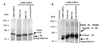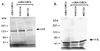Proteasome-dependent degradation of the human estrogen receptor - PubMed (original) (raw)
Proteasome-dependent degradation of the human estrogen receptor
Z Nawaz et al. Proc Natl Acad Sci U S A. 1999.
Abstract
In eukaryotic cells, the ubiquitin-proteasome pathway is the major mechanism for the targeted degradation of proteins with short half-lives. The covalent attachment of ubiquitin to lysine residues of targeted proteins is a signal for the recognition and rapid degradation by the proteasome, a large multi-subunit protease. In this report, we demonstrate that the human estrogen receptor (ER) protein is rapidly degraded in mammalian cells in an estradiol-dependent manner. The treatment of mammalian cells with the proteasome inhibitor MG132 inhibits activity of the proteasome and blocks ER degradation, suggesting that ER protein is turned over through the ubiquitin-proteasome pathway. In addition, we show that in vitro ER degradation depends on ubiquitin-activating E1 enzyme (UBA) and ubiquitin-conjugating E2 enzymes (UBCs), and the proteasome inhibitors MG132 and lactacystin block ER protein degradation in vitro. Furthermore, the UBA/UBCs and proteasome inhibitors promote the accumulation of higher molecular weight forms of ER. The UBA and UBCs, which promote ER degradation in vitro, have no significant effect on human progesterone receptor and human thyroid hormone receptor beta proteins.
Figures
Figure 1
ER degradation depends on the ubiquitin–proteasome pathway. HeLa cells were transiently transfected with 4 ng of pCMV5hER and 750 ng of pERE.E1b.LUC. The cells were treated with either vehicle (DMSO) or proteasome inhibitor (1 μM MG132) both in the absence (−) and presence of 10−9 M estradiol (E2). The ER expression was analyzed by Western blot by using an anti-ER antibody, H222.
Figure 2
In vitro ER degradation depends on ubiquitin pathway enzymes, UBA and UBCs. 35S-labeled ER protein was synthesized in vitro with TNT-coupled rabbit reticulocyte extracts. The labeled ER protein was incubated with ATP and ubiquitin either in the absence of UBA/UBCs (for 120 min) or presence of bacterially expressed UBA and UBCs (UbcH5B and UbcH7). Reactions were terminated at varying times by adding SDS-loading buffer and analyzed by SDS/PAGE and autoradiography. Arrows indicate the position of intact and degraded ER protein.
Figure 3
The proteasome inhibitors, MG132 and lactacystin, block ER degradation in vitro. (A) 35S-labeled ER protein was synthesized in vitro in the presence of either vehicle only, 33 μM MG132 or 33 μM lactacystin with TNT-coupled rabbit reticulocyte extracts. The labeled ER protein was then incubated with ATP and ubiquitin either in the absence of UBA/UBCs or in the presence of UBA and UBCs (UbcH5B and UbcH7). Arrows indicate the position of intact and degraded ER protein. (B) The UBA/UBCs and proteasome inhibitors, MG132 and lactacystin, promote the accumulation of slower migrating forms of ER (shown by a bracket). 35S-labeled ER protein was synthesized in vitro in the presence of either vehicle only, 33 μM MG132 or 33 μM lactacystin with TNT-coupled rabbit reticulocyte extracts. The labeled ER protein was then incubated with ATP and ubiquitin either in the absence of UBA/UBCs or in the presence of UBA and UBCs (UbcH5B and UbcH7). Then the ER protein was analyzed by Western blot analysis using H222 antibody that specifically recognizes ER. The control lane contains reticulocyte extract only. Arrows indicate the position of intact, degraded, and slower migrating forms of ER protein.
Figure 4
PR and TR proteins are not the target of the ubiquitin–proteasome pathway. (A) 35S-labeled PR protein was incubated with ATP and ubiquitin either in the absence of UBA/UBCs or in the presence of UBA and UBCs for 120 min (UbcH5B and UbcH7). In the presence of UBA/UBCs, the reaction mixtures were treated with either vehicle or 33 μM MG132. The position of intact PR is indicated by the arrow. (B) 35S-labeled TR was incubated with ATP and ubiquitin either in the absence of UBA/UBCs or in the presence of UBA and UBCs for 120 min (UbcH5B and UbcH7). The reaction mixtures containing UBA and UBCs were treated with either vehicle or 33 μM MG132. The position of intact TR is indicated by the arrow.
Similar articles
- CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway.
Schubert U, Antón LC, Bacík I, Cox JH, Bour S, Bennink JR, Orlowski M, Strebel K, Yewdell JW. Schubert U, et al. J Virol. 1998 Mar;72(3):2280-8. doi: 10.1128/JVI.72.3.2280-2288.1998. J Virol. 1998. PMID: 9499087 Free PMC article. - The degradation of nascent fibrinogen chains is mediated by the ubiquitin proteasome pathway.
Xia H, Redman C. Xia H, et al. Biochem Biophys Res Commun. 1999 Aug 11;261(3):590-7. doi: 10.1006/bbrc.1999.1081. Biochem Biophys Res Commun. 1999. PMID: 10441471 - The ubiquitin-proteasome pathway and proteasome inhibitors.
Myung J, Kim KB, Crews CM. Myung J, et al. Med Res Rev. 2001 Jul;21(4):245-73. doi: 10.1002/med.1009. Med Res Rev. 2001. PMID: 11410931 Free PMC article. Review. - What do we really know about the ubiquitin-proteasome pathway in muscle atrophy?
Jagoe RT, Goldberg AL. Jagoe RT, et al. Curr Opin Clin Nutr Metab Care. 2001 May;4(3):183-90. doi: 10.1097/00075197-200105000-00003. Curr Opin Clin Nutr Metab Care. 2001. PMID: 11517350 Review.
Cited by
- Proximal and distal regulation of the HYAL1 gene cluster by the estrogen receptor α in breast cancer cells.
Edjekouane L, Benhadjeba S, Jangal M, Fleury H, Gévry N, Carmona E, Tremblay A. Edjekouane L, et al. Oncotarget. 2016 Nov 22;7(47):77276-77290. doi: 10.18632/oncotarget.12630. Oncotarget. 2016. PMID: 27764788 Free PMC article. - Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span.
Schwarz JM, Nugent BM, McCarthy MM. Schwarz JM, et al. Endocrinology. 2010 Oct;151(10):4871-81. doi: 10.1210/en.2010-0142. Epub 2010 Aug 11. Endocrinology. 2010. PMID: 20702577 Free PMC article. - Selective estrogen receptor modulators 4-hydroxytamoxifen and raloxifene impact the stability and function of SRC-1 and SRC-3 coactivator proteins.
Lonard DM, Tsai SY, O'Malley BW. Lonard DM, et al. Mol Cell Biol. 2004 Jan;24(1):14-24. doi: 10.1128/MCB.24.1.14-24.2004. Mol Cell Biol. 2004. PMID: 14673139 Free PMC article. - A first order phase transition mechanism underlies protein aggregation in mammalian cells.
Narayanan A, Meriin A, Andrews JO, Spille JH, Sherman MY, Cisse II. Narayanan A, et al. Elife. 2019 Feb 4;8:e39695. doi: 10.7554/eLife.39695. Elife. 2019. PMID: 30716021 Free PMC article. - 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (p,p'-DDE) disrupts the estrogen-androgen balance regulating the growth of hormone-dependent breast cancer cells.
Aubé M, Larochelle C, Ayotte P. Aubé M, et al. Breast Cancer Res. 2008;10(1):R16. doi: 10.1186/bcr1862. Epub 2008 Feb 14. Breast Cancer Res. 2008. PMID: 18275596 Free PMC article.
References
- Pickart C M. FASEB J. 1997;11:1055–1066. - PubMed
- Haas A L, Siepmann T J. FASEB J. 1997;11:1257–1268. - PubMed
- Chen Z J, Parent L, Maniatis T. Cell. 1996;84:853–862. - PubMed
- Kim T K, Maniatis T. Science. 1996;273:1717–1719. - PubMed
- Ciechanover A, Schwartz A L. FASEB J. 1994;8:182–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials