Systemic administration of interleukin 2 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines - PubMed (original) (raw)
Systemic administration of interleukin 2 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines
K Shimizu et al. Proc Natl Acad Sci U S A. 1999.
Abstract
We have reported previously that murine bone marrow-derived dendritic cells (DC) pulsed with whole tumor lysates can mediate potent antitumor immune responses both in vitro and in vivo. Because successful therapy was dependent on host immune T cells, we have now evaluated whether the systemic administration of the T cell stimulatory/growth promoting cytokine interleukin-2 (IL-2) could enhance tumor lysate-pulsed DC-based immunizations to further promote protective immunity toward, and therapeutic rejection of, syngeneic murine tumors. In three separate approaches using a weakly immunogenic sarcoma (MCA-207), the systemic administration of nontoxic doses of recombinant IL-2 (20,000 and 40,000 IU/dose) was capable of mediating significant increases in the potency of DC-based immunizations. IL-2 could augment the efficacy of tumor lysate-pulsed DC to induce protective immunity to lethal tumor challenge as well as enhance splenic cytotoxic T lymphocyte activity and interferon-gamma production in these treated mice. Moreover, treatment with the combination of tumor lysate-pulsed DC and IL-2 could also mediate regressions of established pulmonary 3-day micrometastases and 7-day macrometastases as well as established 14- and 28-day s.c. tumors, leading to either significant cure rates or prolongation in overall survival. Collectively, these findings show that nontoxic doses of recombinant IL-2 can potentiate the antitumor effects of tumor lysate-pulsed DC in vivo and provide preclinical rationale for the use of IL-2 in DC-based vaccine strategies in patients with advanced cancer.
Figures
Figure 1
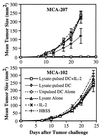
Systemic administration of IL-2 can enhance the efficacy of tumor-lysate pulsed DC to induce specific protective immunity to lethal tumor challenge. As described, mice were immunized twice s.c. in the right flank with MCA-207 tumor lysate-pulsed DC and received i.p. injections of IL-2 after each immunization. Control groups of mice received either HBSS, tumor lysate alone, unpulsed DC alone, MCA-207 tumor lysate-pulsed DC alone, or IL-2 alone. All mice were then rechallenged with a lethal dose of either MCA-207 (Upper) or the unrelated MCA-102 (Lower) viable tumor cells by s.c. injection in the left flank. Data are reported as the average tumor area ± SEM of five or more mice/group.
Figure 2
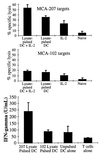
Systemic administration of IL-2 can enhance the capacity of MCA-207 tumor lysate-pulsed DC to induce tumor-specific CTL (Top and Middle) and IFN-γ production (Bottom) in vivo. Mice were immunized twice weekly following the schedule described in Fig. 1. Spleens were harvested 7 days after the final IL-2 (or HBSS) injection from mice treated with either HBSS, MCA-207 tumor lysate-pulsed DC plus IL-2, MCA-207 tumor lysate-pulsed DC alone, or IL-2 alone. Details of the generation and testing of these CTL are provided in Materials and Methods. Values are the mean ± SEM of triplicate wells at a 100:1 effector-to-target ratio. For measurement of IFN-γ production (Bottom), splenic T cells from the treated mice were stimulated in vitro as described. Culture supernatants were harvested 48 hr later and evaluated for IFN-γ levels by ELISA (in units/ml; mean ± SEM of triplicate samples).
Figure 3
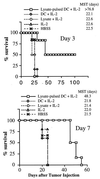
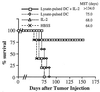
Treatment with the combination of tumor lysate-pulsed DC and IL-2 can prolong survival and result in cures of mice bearing established pulmonary metastases. Mice harboring MCA-207 lung metastases were immunized three times on days 3, 7, and 11 (Upper) or days 7, 10, and 13 (Lower) after tumor injection. IL-2 was given i.p. twice daily at 20,000 IU/dose (Upper) or 40,000 IU/dose (Lower) for 3 consecutive days after each immunization. Control groups of mice received HBSS, tumor lysate plus IL-2, unpulsed DC plus IL-2, or IL-2 alone. Survival was monitored over time after tumor injection, and the MST (in days) was determined.
Figure 4
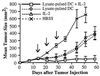
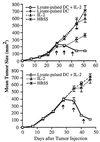
Treatment of mice with tumor lysate-pulsed DC and the systemic administration of IL-2 results in regression of established, 14-day s.c. tumor. A total of 2 × 105 syngeneic MCA-207 sarcoma cells were injected s.c. in the right flank. Mice bearing 14-day tumors were then treated weekly three times with 1 × 106 MCA-207 tumor lysate-pulsed DC in the left flank. IL-2 was given i.p. twice daily at 20,000 IU/dose for 5 consecutive days after each immunization. Control groups of mice received HBSS, MCA-207 tumor lysate-pulsed DC alone, or IL-2 alone. The size of the tumors was assessed in a blinded, coded fashion twice weekly and recorded as tumor area (in mm2).
Figure 5
Combination therapy with tumor lysate-pulsed DC plus IL-2 can prolong survival and result in cures of mice bearing established, 14-day s.c. tumor. Mice were treated 14 days after tumor injection as described in Fig. 4. Survival was monitored over time after tumor injection, and the MST (in days) was determined.
Figure 6
Treatment of mice with tumor lysate-pulsed DC and the systemic administration of IL-2 results in partial regression of established, 28-day s.c. tumor. Mice were treated 28 days after tumor injection as described in Fig. 4. The size of the tumors was assessed in a coded, blinded fashion and recorded as tumor area (in mm2) by measuring the largest perpendicular diameters. Data are reported as the average tumor area ± SEM of five or more mice/group.
Similar articles
- Potentiation of immunologic responsiveness to dendritic cell-based tumor vaccines by recombinant interleukin-2.
Shimizu K, Fields RC, Redman BG, Giedlin M, Mulé JJ. Shimizu K, et al. Cancer J Sci Am. 2000 Feb;6 Suppl 1:S67-75. Cancer J Sci Am. 2000. PMID: 10685663 - Pulsing of dendritic cells with cell lysates from either B16 melanoma or MCA-106 fibrosarcoma yields equally effective vaccines against B16 tumors in mice.
DeMatos P, Abdel-Wahab Z, Vervaert C, Hester D, Seigler H. DeMatos P, et al. J Surg Oncol. 1998 Jun;68(2):79-91. doi: 10.1002/(sici)1096-9098(199806)68:2<79::aid-jso3>3.0.co;2-h. J Surg Oncol. 1998. PMID: 9624036 Review. - Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines.
Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Zitvogel L, et al. J Exp Med. 1996 Jan 1;183(1):87-97. doi: 10.1084/jem.183.1.87. J Exp Med. 1996. PMID: 8551248 Free PMC article. - Augmentation of antitumor immune responses after adoptive transfer of bone marrow derived from donors immunized with tumor lysate-pulsed dendritic cells.
Asavaroengchai W, Kotera Y, Koike N, Pilon-Thomas S, Mulé JJ. Asavaroengchai W, et al. Biol Blood Marrow Transplant. 2004 Aug;10(8):524-33. doi: 10.1016/j.bbmt.2004.04.003. Biol Blood Marrow Transplant. 2004. PMID: 15282530 - Generating a T cell tumor-specific immune response in vivo: can flt3-ligand-generated dendritic cells tip the balance?
McKenna HJ. McKenna HJ. Cancer Immunol Immunother. 1999 Sep;48(6):281-6. doi: 10.1007/s002620050576. Cancer Immunol Immunother. 1999. PMID: 10473802 Free PMC article. Review.
Cited by
- Advancements in dendritic cell vaccination: enhancing efficacy and optimizing combinatorial strategies for the treatment of glioblastoma.
Subtirelu RC, Teichner EM, Ashok A, Parikh C, Talasila S, Matache IM, Alnemri AG, Anderson V, Shahid O, Mannam S, Lee A, Werner T, Revheim ME, Alavi A. Subtirelu RC, et al. Front Neurol. 2023 Oct 31;14:1271822. doi: 10.3389/fneur.2023.1271822. eCollection 2023. Front Neurol. 2023. PMID: 38020665 Free PMC article. Review. - Regulation of dendritic cell interleukin-12 secretion by tumour cell necrosis.
Kandil H, Bachy V, Williams DJ, Helmi R, Gotch FM, Ibrahim MA. Kandil H, et al. Clin Exp Immunol. 2005 Apr;140(1):54-64. doi: 10.1111/j.1365-2249.2005.02730.x. Clin Exp Immunol. 2005. PMID: 15762875 Free PMC article. - Dendritic cell-based immunotherapy of renal cell carcinoma.
Gitlitz BJ, Figlin RA, Pantuck AJ, Belldegrun AS. Gitlitz BJ, et al. Curr Urol Rep. 2001 Feb;2(1):46-52. doi: 10.1007/s11934-001-0025-9. Curr Urol Rep. 2001. PMID: 12084295 Review. - Autologous dendritic cell based adoptive immunotherapy of patients with colorectal cancer-A phase I-II study.
Hunyadi J, András C, Szabó I, Szántó J, Szluha K, Sipka S, Kovács P, Kiss A, Szegedi G, Altorjay I, Sápy P, Antal-Szalmás P, Tóth L, Fazekas G, Rajnavölgyi É. Hunyadi J, et al. Pathol Oncol Res. 2014 Apr;20(2):357-65. doi: 10.1007/s12253-013-9704-3. Epub 2013 Oct 28. Pathol Oncol Res. 2014. PMID: 24163303 Clinical Trial. - Phase Ib trial assessing autologous, tumor-pulsed dendritic cells as a vaccine administered with or without IL-2 in patients with metastatic melanoma.
Redman BG, Chang AE, Whitfield J, Esper P, Jiang G, Braun T, Roessler B, Mulé JJ. Redman BG, et al. J Immunother. 2008 Jul-Aug;31(6):591-8. doi: 10.1097/CJI.0b013e31817fd90b. J Immunother. 2008. PMID: 18528294 Free PMC article. Clinical Trial.
References
- Steinman R M. Annu Rev Immunol. 1991;9:271–296. - PubMed
- Hart D N C. Blood. 1997;90:3245–3287. - PubMed
- Cohen P J, Cohen P A, Rosenberg S A, Katz S I, Mulé J J. Eur J Immunol. 1994;24:315–319. - PubMed
- Cohen P A, Cohen P J, Rosenberg S A, Mulé J J. Cancer Res. 1994;54:1055–1058. - PubMed
- Geraghty P J, Fields R C, Mulé J J. Surg Forum. 1996;47:459–461.
Publication types
MeSH terms
Substances
Grants and funding
- M01-RR00042/RR/NCRR NIH HHS/United States
- 1 R01 CA71669/CA/NCI NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- P01 CA059327/CA/NCI NIH HHS/United States
- 5 P01 CA59327/CA/NCI NIH HHS/United States
- R01 CA071669/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical