Ectopic bone morphogenetic proteins 5 and 4 in the chicken forebrain lead to cyclopia and holoprosencephaly - PubMed (original) (raw)
Ectopic bone morphogenetic proteins 5 and 4 in the chicken forebrain lead to cyclopia and holoprosencephaly
J A Golden et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Proper dorsal-ventral patterning in the developing central nervous system requires signals from both the dorsal and ventral portions of the neural tube. Data from multiple studies have demonstrated that bone morphogenetic proteins (BMPs) and Sonic hedgehog protein are secreted factors that regulate dorsal and ventral specification, respectively, within the caudal neural tube. In the developing rostral central nervous system Sonic hedgehog protein also participates in ventral regionalization; however, the roles of BMPs in the developing brain are less clear. We hypothesized that BMPs also play a role in dorsal specification of the vertebrate forebrain. To test our hypothesis we implanted beads soaked in recombinant BMP5 or BMP4 into the neural tube of the chicken forebrain. Experimental embryos showed a loss of the basal telencephalon that resulted in holoprosencephaly (a single cerebral hemisphere), cyclopia (a single midline eye), and loss of ventral midline structures. In situ hybridization using a panel of probes to genes expressed in the dorsal and ventral forebrain revealed the loss of ventral markers with the maintenance of dorsal markers. Furthermore, we found that the loss of the basal telencephalon was the result of excessive cell death and not a change in cell fates. These data provide evidence that BMP signaling participates in dorsal-ventral patterning of the developing brain in vivo, and disturbances in dorsal-ventral signaling result in specific malformations of the forebrain.
Figures
Figure 1
Whole-mount in situ hybridization with a probe to chicken BMP5. (A) Staining of E4.5 embryos (implanted with a bead soaked in buffer alone) shows the normal expression pattern of BMP5 along the dorsal aspect of the brain, including the diencephalon (upper arrow) and the telencephalon (lower arrow). The expression was through the entire wall of the neural tube (data not shown). No expression was seen in the ventral forebrain (data not shown). Expression persists in embryos exposed to rBMP4 protein (B and C) and may actually be slightly increased (arrowhead points to implanted bead in C; see Materials and Methods), with staining extending laterally in the telencephalon (B). T, telencephalon; D, diencephalon; M, mesencephalon (tectum).
Figure 2
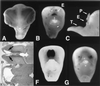
Brain phenotype and spectrum of cyclopia. An E9 control chicken (A) is compared with E9 chicken embryos exposed to rBMP5 (B, C, and G) or rBMP4 (F). Variation from a single midline eye (black region) to small slightly separated eyes was seen in embryos exposed to BMP5 and BMP4. (C) Lateral view of the embryo in B highlights the position of the eye (E) relative to the superiorly placed proboscis (nasal anlagen, P) and virtually absent upper beak, resulting in the easy visualization of the tongue (T). The control E9 chicken brain (D) sectioned in the horizontal plane shows a single third ventricle (right) and two lateral ventricles (arrows, left). A horizontal section from an E9 cyclopic chicken shows a single third ventricle (right) and a single holosphere instead of two lateral ventricles (left). (D and E, ×25.)
Figure 3
In situ hybridization for dorsal and ventral markers in the forebrain of E4 embryos. _Wnt_-1 is expressed over the dorsal midline of the mesencephalon and diencephalon. Expression of _Wnt_-1 is normal or increased in embryos exposed to rBMP4 or rBMP5. _Wnt_-4 expression in the dorsal diencephalon expands ventrally in rBMP4-treated embryos compared with the control embryo. Pax-6, normally expressed in the dorsal telencephalon, is still expressed in the embryo exposed to rBMP4 and rBMP5, although the domain of expression is reduced in embryos exposed to rBMPs. In contrast to the dorsally expressed genes above, Pax-2 expression is completely absent in the ventral eye and ventral forebrain (arrowheads point to same region in each embryo). Nkx-2.1 expression normally seen in the basal forebrain is absent in embryos treated with rBMP4 and rBMP5. The embryonic head has been isolated and photographed from the ventral side. The eye (labeled) can be seen in each image and the rostral (Ro) end of the head is to the right in each image (∗ denotes the implanted bead). Shh expression in the ventral forebrain ends just rostral to the optic chiasm. The morphologically distinct basal telencephalon (arrows) is present rostral to the Shh expression domain. Embryos implanted with beads soaked in rBMP4 or rBMP5 show no morphologically identifiable basal telencephalon; the Shh expression domain comes up to the rostral limit of the ventral brain. All embryos were sectioned to confirm the whole-mount in situ hybridization staining patterns.
Figure 4
Coronal sections of the forebrain showing the expression of _Wnt_-4 by in situ hybridization. (A) Expression in a control embryo on E4.5. (B) Expression in an embryo on E4.5 after implanting a bead soaked in rBMP5. Note the expanded domain of expression after exposure to BMP5. (×200.)
Figure 5
Expression of _Wnt_-4 mRNA after dorsal forebrain extirpation. (A) No expression of _Wnt_-4 is seen after dorsal forebrain removal alone at stage 9 (lateral view; arrow, eye; arrowhead, site where _Wnt_-4 should be expressed). (B) _Wnt_-4 expression is seen after implantation of bead soaked in rBMP5 (blue bead seen in neural tube; white arrows, eyes; black arrows, expression of _Wnt_-4).
Figure 6
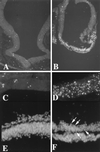
TUNEL assay for cell death. Relatively few cells were labeled by TUNEL in unoperated embryos or embryos implanted with a bead soaked in buffer (A, coronal section of forebrain, dorsal is up and ventral down; ×100). In contrast, a selective and extensive labeling of cells, indicating cell death, was found in the ventral telencephalon after exposure to rBMP5 or rBMP4 proteins (B, coronal section of E3 telencephalon, dorsal is up and ventral down, a bead soaked in rBMP4 protein was implanted at stage 11; ×100). At higher power, the difference between dorsal (C) and ventral (D) telencephalon is striking (both C and D from an embryo implanted at stage 9 with a bead soaked in rBMP5 protein and harvested on E4; ×400). 4′,6-Diamidino-2-phenylindole (DAPI) nuclear stain confirmed the TUNEL findings. (E) Field of dorsal neural tube of embryo exposed to rBMP4 protein shows all intact nuclei. (×400.) (F) A field from the ventral telencephalon of the same section as E shows numerous condensed nuclei (arrows) characteristic of cells undergoing apoptosis. (×400.)
Similar articles
- Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles.
Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Crossley PH, et al. Neuroscience. 2001;108(2):183-206. doi: 10.1016/s0306-4522(01)00411-0. Neuroscience. 2001. PMID: 11734354 - Dorsal-ventral patterning defects in the eye of BF-1-deficient mice associated with a restricted loss of shh expression.
Huh S, Hatini V, Marcus RC, Li SC, Lai E. Huh S, et al. Dev Biol. 1999 Jul 1;211(1):53-63. doi: 10.1006/dbio.1999.9303. Dev Biol. 1999. PMID: 10373304 - Genetic patterning of the mammalian telencephalon by morphogenetic molecules and transcription factors.
Takahashi H, Liu FC. Takahashi H, et al. Birth Defects Res C Embryo Today. 2006 Sep;78(3):256-66. doi: 10.1002/bdrc.20077. Birth Defects Res C Embryo Today. 2006. PMID: 17061260 Review. - Sonic hedgehog: a common signal for ventral patterning along the rostrocaudal axis of the neural tube.
Ericson J, Muhr J, Jessell TM, Edlund T. Ericson J, et al. Int J Dev Biol. 1995 Oct;39(5):809-16. Int J Dev Biol. 1995. PMID: 8645565 Review.
Cited by
- Winding through the WNT pathway during cellular development and demise.
Li F, Chong ZZ, Maiese K. Li F, et al. Histol Histopathol. 2006 Jan;21(1):103-24. doi: 10.14670/HH-21.103. Histol Histopathol. 2006. PMID: 16267791 Free PMC article. Review. - Essential roles of mesenchyme-derived beta-catenin in mouse Müllerian duct morphogenesis.
Deutscher E, Hung-Chang Yao H. Deutscher E, et al. Dev Biol. 2007 Jul 15;307(2):227-36. doi: 10.1016/j.ydbio.2007.04.036. Epub 2007 May 3. Dev Biol. 2007. PMID: 17532316 Free PMC article. - Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly.
Chen L, Liao G, Yang L, Campbell K, Nakafuku M, Kuan CY, Zheng Y. Chen L, et al. Proc Natl Acad Sci U S A. 2006 Oct 31;103(44):16520-5. doi: 10.1073/pnas.0603533103. Epub 2006 Oct 18. Proc Natl Acad Sci U S A. 2006. PMID: 17050694 Free PMC article. - Natural bone fragmentation in the blind cave-dwelling fish, Astyanax mexicanus: candidate gene identification through integrative comparative genomics.
Gross JB, Stahl BA, Powers AK, Carlson BM. Gross JB, et al. Evol Dev. 2016 Jan-Feb;18(1):7-18. doi: 10.1111/ede.12131. Epub 2015 Jul 8. Evol Dev. 2016. PMID: 26153732 Free PMC article. - Altered gene expression with abnormal patterning of the telencephalon in embryos of diabetic Albino Swiss mice.
Liao DM, Ng YK, Tay SSW, Ling EA, Dheen ST. Liao DM, et al. Diabetologia. 2004 Mar;47(3):523-531. doi: 10.1007/s00125-004-1351-5. Epub 2004 Feb 13. Diabetologia. 2004. PMID: 14963649
References
- Rubenstein J L, Beachy P A. Curr Opin Neurobiol. 1998;8:18–26. - PubMed
- Echelard Y, Epstein D J, St-Jacques B, Shen L, Mohler J, McMahon J A, McMahon A P. Cell. 1993;75:1417–1430. - PubMed
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell T M. Cell. 1996;87:661–673. - PubMed
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell T M, et al. Cell. 1994;76:761–775. - PubMed
- Roelink H, Porter J A, Chiang C, Tanabe Y, Chang D T, Beachy P A, Jessell T M. Cell. 1995;81:445–455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources