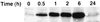Epithelial sodium channel regulated by aldosterone-induced protein sgk - PubMed (original) (raw)
Epithelial sodium channel regulated by aldosterone-induced protein sgk
S Y Chen et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Sodium homeostasis in terrestrial and freshwater vertebrates is controlled by the corticosteroid hormones, principally aldosterone, which stimulate electrogenic Na+ absorption in tight epithelia. Although aldosterone is known to increase apical membrane Na+ permeability in target cells through changes in gene transcription, the mechanistic basis of this effect remains poorly understood. The predominant early effect of aldosterone is to increase the activity of the epithelial sodium channel (ENaC), although ENaC mRNA and protein levels do not change initially. Rather, the open probability and/or number of channels in the apical membrane are greatly increased by unknown modulators. To identify hormone-stimulated gene products that modulate ENaC activity, a subtracted cDNA library was generated from A6 cells, a stable cell line of renal distal nephron origin, and the effect of candidates on ENaC activity was tested in a coexpression assay. We report here the identification of sgk (serum and glucocorticoid-regulated kinase), a member of the serine-threonine kinase family, as an aldosterone-induced regulator of ENaC activity. sgk mRNA and protein were strongly and rapidly hormone stimulated both in A6 cells and in rat kidney. Furthermore, sgk stimulated ENaC activity approximately 7-fold when they were coexpressed in Xenopus laevis oocytes. These data suggest that sgk plays a central role in aldosterone regulation of Na+ absorption and thus in the control of extracellular fluid volume, blood pressure, and sodium homeostasis.
Figures
Figure 1
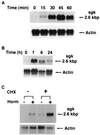
sgk mRNA is rapidly and directly stimulated by dexamethasone in A6 cells. A6 cells were grown on permeable supports, and parallel cultures were treated with vehicle or 10−7 M dexamethasone for the times shown. Blots were hybridized with a probe for Xenopus sgk, stripped, and rehybridized with a probe for Xenopus type 8 actin. (A and B) Northern blots representing 1- and 24-h time courses of hormone treatment, respectively. In four separate experiments for each time course, fold activation (quantitated by PhosphorImager) varied by <20% at each time. sgk message constituted a single major band on Northern blot with a size of 2.6 kbp. (C) Hormone stimulation of sgk does not require new protein synthesis. A6 cells were incubated with cycloheximide (CHX) for 2 h and then cells were treated with vehicle or 10−7 M dexamethasone for 2 h additional, as shown. Cycloheximide blocked hormone induction of PD/R by 90% (not shown), as described (22). The blot is representative of three independent experiments with similar results.
Figure 2
sgk immunoreactive protein is rapidly stimulated by dexamethasone in A6 cells. A6 cells were grown on permeable supports and treated with 10−7 M dexamethasone for the times shown. Immunoblots were prepared and probed as described in Materials and Methods. The blot shown is representative of four different blots from two independent experiments performed on different days. A single major band of approximately 56 kDa was observed.
Figure 3
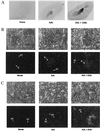
In situ hybridization of sgk in rat kidney. Adx rats were treated for 4 h with vehicle (Adx, n = 5) or aldosterone (Adx + Aldo, n = 5). Longitudinal frozen sections (15 μm) were cut and hybridized with a rat sgk RNA probe (see Materials and Methods). (A) Autoradiagram of whole kidney. (B–F) Emulsion-dipped sections counterstained with hematoxylin and eosin at ×200 magnification (except D, which is ×400). A darkfield view is shown immediately beneath each light micrograph. Sense controls performed on sections from the adx group are shown for each kidney region. Sense controls for adx + aldo showed similar levels of signal (not shown). (B) Glomerulus (G). (C) Cortical tubules [proximal tubule (PT); distal tubule/collecting duct (DT)]. (D) ×400 magnification of cortex showing a tubule with morphology consistent with distal nephron. (E) Outer medulla. (F) Inner medulla/papilla.
Figure 3
In situ hybridization of sgk in rat kidney. Adx rats were treated for 4 h with vehicle (Adx, n = 5) or aldosterone (Adx + Aldo, n = 5). Longitudinal frozen sections (15 μm) were cut and hybridized with a rat sgk RNA probe (see Materials and Methods). (A) Autoradiagram of whole kidney. (B–F) Emulsion-dipped sections counterstained with hematoxylin and eosin at ×200 magnification (except D, which is ×400). A darkfield view is shown immediately beneath each light micrograph. Sense controls performed on sections from the adx group are shown for each kidney region. Sense controls for adx + aldo showed similar levels of signal (not shown). (B) Glomerulus (G). (C) Cortical tubules [proximal tubule (PT); distal tubule/collecting duct (DT)]. (D) ×400 magnification of cortex showing a tubule with morphology consistent with distal nephron. (E) Outer medulla. (F) Inner medulla/papilla.
Figure 4
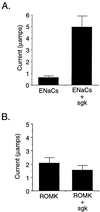
(A) ENaC activity is stimulated by sgk when they are coexpressed in X. laevis oocytes. In vitro transcribed RNA for each of the three Xenopus ENaC subunits was injected into oocytes with or without sgk. Shown is amiloride-sensitive current from a representative experiment with six oocytes per condition (mean ± SE). The mean increase in amiloride-inhibitable sodium current was 6.9-fold ± 1.5 for 12 experiments (six oocytes per condition in each experiment) performed with oocytes from six different frogs. (B) Potassium channel ROMK2 is not stimulated by sgk. Experiments were performed in Xenopus oocytes as in A. Shown is a representative experiment with six oocytes per condition. In two independent experiments performed on different days (six oocytes per condition each), the average ROMK currents were: ROMK alone, 2.06 ± 0.41 μA; ROMK + sgk, 1.52 ± 0.35 μA (ratio ROMK + sgk/ROMK alone = 0.74). In the same set of experiments, ENaC controls were performed and currents were: ENaC alone, 0.442 ± 0.125 μA; ENaC + sgk, 3.2 ± 0.59 μA (ratio ENaC + sgk/ENaC alone = 7.3).
Similar articles
- Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK.
Loffing J, Zecevic M, Féraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Loffing J, et al. Am J Physiol Renal Physiol. 2001 Apr;280(4):F675-82. doi: 10.1152/ajprenal.2001.280.4.F675. Am J Physiol Renal Physiol. 2001. PMID: 11249859 - Regulation of sgk by aldosterone and its effects on the epithelial Na(+) channel.
Shigaev A, Asher C, Latter H, Garty H, Reuveny E. Shigaev A, et al. Am J Physiol Renal Physiol. 2000 Apr;278(4):F613-9. doi: 10.1152/ajprenal.2000.278.4.F613. Am J Physiol Renal Physiol. 2000. PMID: 10751222 - Role of SGK in mineralocorticoid-regulated sodium transport.
Pearce D, Verrey F, Chen SY, Mastroberardino L, Meijer OC, Wang J, Bhargava A. Pearce D, et al. Kidney Int. 2000 Apr;57(4):1283-9. doi: 10.1046/j.1523-1755.2000.00963.x. Kidney Int. 2000. PMID: 10760055 Review. - The sgk, an aldosterone-induced gene in mineralocorticoid target cells, regulates the epithelial sodium channel.
Náray-Fejes-Tóth A, Fejes-Tóth G. Náray-Fejes-Tóth A, et al. Kidney Int. 2000 Apr;57(4):1290-4. doi: 10.1046/j.1523-1755.2000.00964.x. Kidney Int. 2000. PMID: 10760056 Review. - The serum and glucocorticoid kinase sgk increases the abundance of epithelial sodium channels in the plasma membrane of Xenopus oocytes.
Alvarez de la Rosa D, Zhang P, Náray-Fejes-Tóth A, Fejes-Tóth G, Canessa CM. Alvarez de la Rosa D, et al. J Biol Chem. 1999 Dec 31;274(53):37834-9. doi: 10.1074/jbc.274.53.37834. J Biol Chem. 1999. PMID: 10608847
Cited by
- Nedd4-2 and the regulation of epithelial sodium transport.
Rotin D, Staub O. Rotin D, et al. Front Physiol. 2012 Jun 21;3:212. doi: 10.3389/fphys.2012.00212. eCollection 2012. Front Physiol. 2012. PMID: 22737130 Free PMC article. - Sodium-retaining effect of insulin in diabetes.
Brands MW, Manhiani MM. Brands MW, et al. Am J Physiol Regul Integr Comp Physiol. 2012 Dec;303(11):R1101-9. doi: 10.1152/ajpregu.00390.2012. Epub 2012 Oct 3. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 23034715 Free PMC article. Review. - Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha.
Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC. Zhang W, et al. J Clin Invest. 2007 Mar;117(3):773-83. doi: 10.1172/JCI29850. J Clin Invest. 2007. PMID: 17332896 Free PMC article. - Hrs controls sorting of the epithelial Na+ channel between endosomal degradation and recycling pathways.
Zhou R, Kabra R, Olson DR, Piper RC, Snyder PM. Zhou R, et al. J Biol Chem. 2010 Oct 1;285(40):30523-30. doi: 10.1074/jbc.M110.150755. Epub 2010 Jul 30. J Biol Chem. 2010. PMID: 20675381 Free PMC article. - Antiviral activity of the mineralocorticoid receptor NR3C2 against Herpes simplex virus Type 1 (HSV-1) infection.
Haas JG, Weber J, Gonzalez O, Zimmer R, Griffiths SJ. Haas JG, et al. Sci Rep. 2018 Oct 26;8(1):15876. doi: 10.1038/s41598-018-34241-w. Sci Rep. 2018. PMID: 30367157 Free PMC article.
References
- Smith H W. From Fish to Philosopher. Garden City, NY: Doubleday; 1961.
- Edelman I S, Fimognari G M. Recent Prog Horm Res. 1968;24:1–44. - PubMed
- Eaton D C, Becchetti A, Ma H, Ling B N. Kidney Int. 1995;48:941–949. - PubMed
- Kemendy A E, Kleyman T R, Eaton D C. Am J Physiol. 1992;263:C825–C837. - PubMed
- Kleyman T R, Coupaye G B, Ernst S A. J Biol Chem. 1992;267:9622–9628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases