Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons - PubMed (original) (raw)
Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons
S J Mowla et al. J Neurosci. 1999.
Abstract
Nerve growth factor (NGF) is released through the constitutive secretory pathway from cells in peripheral tissues and nerves where it can act as a target-derived survival factor. In contrast, brain-derived neurotrophic factor (BDNF) appears to be processed in the regulated secretory pathway of brain neurons and secreted in an activity-dependent manner to play a role in synaptic plasticity. To determine whether sorting differences are intrinsic to the neurotrophins or reflect differences between cell types, we compared NGF and BDNF processing in cultured hippocampal neurons using a Vaccinia virus expression system. Three independent criteria (retention or release from cells after pulse-chase labeling, depolarization-dependent release, and immunocytochemical localization) suggest that the bulk of newly synthesized NGF is sorted into the constitutive pathway, whereas BDNF is primarily sorted into the regulated secretory pathway. Similar results occurred with AtT 20 cells, including those transfected with cDNAs encoding neurotrophin precursor-green fluorescent protein fusions. The NGF precursor, but not the BDNF precursor, is efficiently cleaved by the endoprotease furin in the trans-Golgi network (TGN). Blocking furin activity in AtT 20 cells with alpha1-PDX as well as increasing the expression of NGF precursor partially directed NGF into the regulated secretory pathway. Therefore, neurotrophins can be sorted into either the constitutive or regulated secretory pathways, and sorting may be regulated by the efficiency of furin cleavage in the TGN. This mechanism may explain how neuron-generated neurotrophins can act both as survival factors and as neuropeptides.
Figures
Fig. 1.
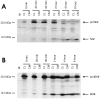
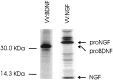
Pulse–chase metabolic labeling of pro-NGF (A) and pro-BDNF (B) in cultures of hippocampal neurons. Separate plates of cells were infected with VV encoding the NGF precursor or the BDNF precursor for 1 hr and postincubated in fresh medium without virus for 10 hr. Cells were exposed to medium containing [35S] Cys-Met for 30 min and chased for 0, 0.5, 1, 2, 4, and 8 hr. Identical volumes (750 μl) of cell lysates (CL) and conditioned media (CM) were immunoprecipitated with antibodies to NGF or BDNF or with nonimmune serum (NI; a cell lysate sample) and electrophoresed on 13–22% SDS gradient gels. Dried gels were exposed to a phosphorimaging screen.
Fig. 2.
Kinetics of NGF and BDNF retention in hippocampal neurons. Experiments in Figure 1 were repeated three times, and the combined results were analyzed by the ImageQuant program. The ratio of mature NGF and BDNF in cell lysates (CL) was compared with the total NGF and BDNF in CL + conditioned medium (CM). Data show that significantly larger amounts of BDNF are retained in CL than NGF.
Fig. 3.
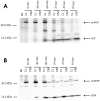
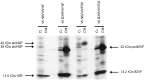
Pulse–chase metabolic labeling of pro-NGF (A) and pro-BDNF (B) production and processing in VV-infected AtT 20 cell cultures. Methods were identical to those described in the legend to Figure 1. NGF and BDNF and their precursors were measured in conditioned medium (CM) and in cell lysates (CL).NI is a sample of CL precipitated with nonimmune serum.
Fig. 4.
Pulse–chase metabolic labeling of primary rat Schwann cells infected with VV encoding pro-BDNF. Methods were identical to those described in the legend to Figure 1. BDNF and its precursor were measured in cell lysates (CL) and conditioned media (CM). NI is a_CL_ sample precipitated with nonimmune serum.
Fig. 5.
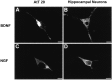
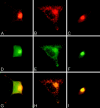
Confocal microscopy of AtT 20 cells (A, C) and hippocampal neurons (B, D) infected with VV encoding pro-NGF (C, D) or pro-BDNF (A,B). Cells were infected for 1 hr and postincubated in the absence of virus for another 8 hr. The cultures were fixed and treated with antibodies against NGF or BDNF, followed by CY3-conjugated goat anti-rabbit IgG. Scale bar, 10 μm.
Fig. 6.
Double-label immunocytochemistry comparing the distribution in infected AtT 20 cells of BDNF and NGF immunoreactivity with immunostaining of endogenous TGN38 and ACTH. BDNF immunoreactivity (A, B) colocalizes with TGN38 (D) in the perinuclear region and with ACTH in the cytoplasm and cell processes (E). G is a composite of A and D; H is a composite of B and E. NGF immunoreactivity (C) colocalizes with TGN38 (F) in the perinuclear region but is not detectable in cell processes. I is a composite of_C_ and F.
Fig. 7.
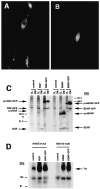
Expression of pro-neurotrophin–GFP fusion proteins in AtT 20 cells. Cells were plated on poly-
l
-lysine-coated coverslips and transfected using lipofectamine with cDNAs encoding either (A) pro-NGF–GFP or (B) pro-BDNF–GFP. Three days later, the cells were analyzed by fluorescence microscopy.C, Immunoprecipitation and SDS-PAGE of metabolically labeled GFP fusion proteins from transfected COS 7 cells.D, Conditioned medium from pro-NGF–GFP or pro-BDNF–GFP expressing COS 7 cells activate Trk A and Trk B phosphorylation, respectively, in NIH 3T3 cells engineered to overexpress either receptor.
Fig. 8.
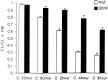
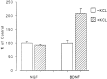
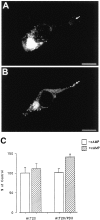
KCl-induced release of BDNF but not NGF from hippocampal neurons. Hippocampal neurons from E18 mice were cultured for 7 d and infected for 1 hr with VV encoding pro-NGF or pro-BDNF. After 10 hr in medium without virus, the cells were labeled for 30 min with [35S] Cys-Met, incubated in medium without radiolabel for 4 hr, and treated with medium with or without KCl and CaCl2 for 15 min. Conditioned media were immunoprecipitated with antibodies to NGF or BDNF and electrophoresed on a SDS gel. Results were analyzed on a phosphorimager and are an average (±SEM) of three independent experiments.
Fig. 9.
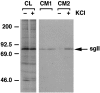
Release of secretogranin II (sgII) from hippocampal neurons infected with VV coding for pro-NGF. Eight hours after neurons were exposed to VV, the cells were pulsed for 30 min with medium containing [35S] Cys-Met. The cells were chased for an additional 4 hr, after which samples of conditioned medium were analyzed (CM1), and again 30 min later after the addition (+) or in the absence (−) of KCl (50 m
m
) added to the culture medium (CM2).
Fig. 10.
Cold-block experiments. Hippocampal neurons were infected with VV encoding pro-NGF or pro-BDNF for 1 hr, and metabolically labeled at 20°C for 3 hr. Cell lysates were prepared, immunoprecipitated with antibodies to NGF or BDNF, and electrophoresed on SDS gels.
Fig. 11.
The effects of α1-PDX on pro-NGF processing in AtT 20 cells. AtT 20 cells were infected for 2 hr with VV encoding pro-NGF or pro-BDNF with or without VV encoding the furin inhibitor α1-PDX. Cells were incubated in virus-free medium for 10 hr and metabolically labeled for 3 hr. Cell lysates (CL) and conditioned media (CM) were collected, immunoprecipitated, and analyzed by SDS gel electrophoresis.
Fig. 12.
The effects of α1-PDX on NGF sorting in AtT 20 cells. AtT 20 cells (A) or AtT 20 cells that stably express α1-PDX (B) were infected for 1 hr with VV encoding pro-NGF, postinfected for 10 hr, and prepared for immunocytochemistry. In C, AtT 20 cells with or without stably expressed α1-PDX were infected with VV encoding pro-NGF for 1 hr, postinfected for 10 hr in control medium, pulsed with medium containing [35S] Cys-Met for 2 hr, chased for 3 hr, and treated with medium with or without 5 m
m
cAMP for 3 hr. Cell lysates and conditioned media were immunoprecipitated and analyzed by SDS-PAGE. Results were analyzed on a phosphorimager and report an average (±SEM) of three independent experiments.
Fig. 13.
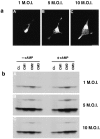
Overexpression of NGF results in missorting of NGF from the constitutive to the regulated secretory pathway.a, AtT 20 cells were infected for 1 hr with 1 (A), 5 (B), or 10 (C) MOI of VV coding for pro-NGF, postinfected for 8 hr, fixed, and prepared for immunocytochemistry using an antibody to NGF followed by a CY3-conjugated secondary antibody. Cells were analyzed by confocal microscopy. b, AtT 20 cells were infected with 1 (A), 5 (B), or 10 (C) MOI for 1 hr followed by a 4 hr postinfection and 3 hr incubation in medium containing [35S] Translabel. Conditioned media were collected (CM1), the cells were chased for 3 hr, and media was again collected (CM2). 8-bromo-cAMP (5 m
m
) was then added to some cultures, and cells were incubated for an additional 3 hr, after which media were collected (CM3) and the cells were lysed (CL). NGF was immunoprecipitated from all samples, and the precipitate was analyzed by SDS-PAGE. Comparison of CM3 samples shows that NGF release can be stimulated by cAMP from cells infected with 5 or 10 MOI but not from cells receiving 1 MOI.
Similar articles
- Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor.
Farhadi HF, Mowla SJ, Petrecca K, Morris SJ, Seidah NG, Murphy RA. Farhadi HF, et al. J Neurosci. 2000 Jun 1;20(11):4059-68. doi: 10.1523/JNEUROSCI.20-11-04059.2000. J Neurosci. 2000. PMID: 10818141 Free PMC article. - Are there differences between the secretion characteristics of NGF and BDNF? Implications for the modulatory role of neurotrophins in activity-dependent neuronal plasticity.
Griesbeck O, Canossa M, Campana G, Gärtner A, Hoener MC, Nawa H, Kolbeck R, Thoenen H. Griesbeck O, et al. Microsc Res Tech. 1999 May 15-Jun 1;45(4-5):262-75. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<262::AID-JEMT10>3.0.CO;2-K. Microsc Res Tech. 1999. PMID: 10383119 - Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 are sorted to dense-core vesicles and released via the regulated pathway in primary rat cortical neurons.
Wu YJ, Krüttgen A, Möller JC, Shine D, Chan JR, Shooter EM, Cosgaya JM. Wu YJ, et al. J Neurosci Res. 2004 Mar 15;75(6):825-34. doi: 10.1002/jnr.20048. J Neurosci Res. 2004. PMID: 14994343 - Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS.
Lessmann V. Lessmann V. Gen Pharmacol. 1998 Nov;31(5):667-74. doi: 10.1016/s0306-3623(98)00190-6. Gen Pharmacol. 1998. PMID: 9809461 Review. - Pro-region of neurotrophins: role in synaptic modulation.
Lu B. Lu B. Neuron. 2003 Aug 28;39(5):735-8. doi: 10.1016/s0896-6273(03)00538-5. Neuron. 2003. PMID: 12948441 Review.
Cited by
- Gangliosides, NGF, brain aging and disease: a mini-review with personal reflections.
Cuello AC. Cuello AC. Neurochem Res. 2012 Jun;37(6):1256-60. doi: 10.1007/s11064-012-0770-9. Epub 2012 Apr 8. Neurochem Res. 2012. PMID: 22484968 Review. - BDNF Induces Striatal-Enriched Protein Tyrosine Phosphatase 61 Degradation Through the Proteasome.
Saavedra A, Puigdellívol M, Tyebji S, Kurup P, Xu J, Ginés S, Alberch J, Lombroso PJ, Pérez-Navarro E. Saavedra A, et al. Mol Neurobiol. 2016 Aug;53(6):4261-4273. doi: 10.1007/s12035-015-9335-7. Epub 2015 Jul 30. Mol Neurobiol. 2016. PMID: 26223799 Free PMC article. - Differential effects of BDNF and neurotrophin 4 (NT4) on endocytic sorting of TrkB receptors.
Proenca CC, Song M, Lee FS. Proenca CC, et al. J Neurochem. 2016 Aug;138(3):397-406. doi: 10.1111/jnc.13676. Epub 2016 Jun 18. J Neurochem. 2016. PMID: 27216821 Free PMC article. - Leukemia inhibitory factor is a key signal for injury-induced neurogenesis in the adult mouse olfactory epithelium.
Bauer S, Rasika S, Han J, Mauduit C, Raccurt M, Morel G, Jourdan F, Benahmed M, Moyse E, Patterson PH. Bauer S, et al. J Neurosci. 2003 Mar 1;23(5):1792-803. doi: 10.1523/JNEUROSCI.23-05-01792.2003. J Neurosci. 2003. PMID: 12629183 Free PMC article. - Huntingtin-associated protein-1 interacts with pro-brain-derived neurotrophic factor and mediates its transport and release.
Wu LL, Fan Y, Li S, Li XJ, Zhou XF. Wu LL, et al. J Biol Chem. 2010 Feb 19;285(8):5614-23. doi: 10.1074/jbc.M109.073197. Epub 2009 Dec 7. J Biol Chem. 2010. PMID: 19996106 Free PMC article.
References
- Acheson A, Barker PA, Alderson RF, Miller FD, Murphy RA. Detection of brain-derived neurotrophic factor-like activity in fibroblasts and Schwann cells: inhibition by antibodies to NGF. Neuron. 1991;7:265–275. - PubMed
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–437. - PubMed
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. - PubMed
- Anderson ED, Thomas L, Hayflick JS, Thomas G. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed α1-antitrypsin variant. J Biol Chem. 1993;268:24887–24891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous