Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine - PubMed (original) (raw)
Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine
J A Parkinson et al. J Neurosci. 1999.
Abstract
Dopamine release within the nucleus accumbens (NAcc) has been associated with both the rewarding and locomotor-stimulant effects of abused drugs. The functions of the NAcc core and shell were investigated in mediating amphetamine-potentiated conditioned reinforcement and locomotion. Rats were initially trained to associate a neutral stimulus (Pavlovian CS) with food reinforcement (US). After excitotoxic lesions that selectively destroyed either the NAcc core or shell, animals underwent additional CS-US training sessions and then were tested for the acquisition of a new instrumental response that produced the CS acting as a conditioned reinforcer (CR). Animals were infused intra-NAcc with D-amphetamine (0, 1, 3, 10, or 20 microg) before each session. Shell lesions affected neither Pavlovian nor instrumental conditioning but completely abolished the potentiative effect of intra-NAcc amphetamine on responding with CR. Core-lesioned animals were impaired during the Pavlovian retraining sessions but showed no deficit in the acquisition of responding with CR. However, the selectivity in stimulant-induced potentiation of the CR lever was reduced, as intra-NAcc amphetamine infusions dose-dependently increased responding on both the CR lever and a nonreinforced (control) lever. Shell lesions produced hypoactivity and attenuated amphetamine-induced activity. In contrast, core lesions resulted in hyperactivity and enhanced the locomotor-stimulating effect of amphetamine. These results indicate a functional dissociation of subregions of the NAcc; the shell is a critical site for stimulant effects underlying the enhancement of responding with CR and locomotion after intra-NAcc injections of amphetamine, whereas the core is implicated in mechanisms underlying the expression of CS-US associations.
Figures
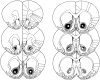
Fig. 1.
Schematic representation of excitotoxic lesions to the NAcc core. Shaded areas represent the smallest (black) and largest (gray) extent of neuronal damage in a single animal. Coronal sections are +2.7 mm anterior through +0.48 mm posterior to bregma (Paxinos and Watson, 1998).
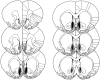
Fig. 2.
Schematic representation of excitotoxic lesions to the NAcc shell. Shaded areas represent the smallest (black) and largest (gray) extent of neuronal damage in a single animal. Coronal sections are +2.7 mm anterior through +0.48 mm posterior to bregma (Paxinos and Watson, 1998).
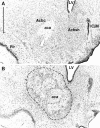
Fig. 3.
Photomicrographs showing cresyl violet-stained coronal sections through the nucleus accumbens (∼+1.2 mm from bregma). A, Sham lesion; B, nucleus accumbens shell lesion. C, High magnification of the sham lesion section shown in A; D, high magnification of the shell lesion section shown in B. The lesioned area is indicated by the dotted lines. Scale bars: A, 1 mm; C, 500 μm.Arrowheads show identical landmarks in _A_and B, and C and D.aca, Anterior commissure; Acbc, nucleus accumbens core; Acbsh, nucleus accumbens shell;LV, lateral ventricle.
Fig. 4.
Photomicrographs showing cresyl violet-stained coronal sections through the nucleus accumbens (∼+1.2 mm from bregma). A, Sham lesion; B, nucleus accumbens core lesion. The lesioned area is indicated by the_dotted lines_. Scale bar: A, 1 mm.aca, Anterior commissure; Acbc, nucleus accumbens core; Acbsh, nucleus accumbens shell;ICjM, major islands of calleja; LV, lateral ventricle; Pir, piriform cortex.
Fig. 5.
Effect of NAcc core and shell lesions on Pavlovian-discriminated approach. The mean ± SEM ratio of approach responses during the CS relative to the CS plus VI period is shown for four presurgical and four postsurgical sessions for core-, shell-, and sham-lesioned animals.
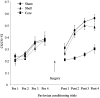
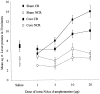
Fig. 6.
Effect of NAcc core lesions on the acquisition of responding with CR. Data points represent the mean square root ± SEM responses on the lever producing the conditioned reinforcer (CR) and the control lever (NCR) for sham- and core-lesioned animals after intra-NAcc injections of
d
-amphetamine (0, 1, 3, 10, and 20 μg).
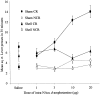
Fig. 7.
Effect of NAcc shell lesions on the acquisition of responding with CR. Data points represent the mean square root ± SEM responses on the lever producing the conditioned reinforcer (CR) and the control lever (NCR) for sham- and shell-lesioned animals after intra-NAcc injections of
d
-amphetamine (0, 1, 3, 10, and 20 μg).
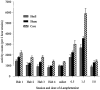
Fig. 8.
Effect of NAcc core and shell lesions on spontaneous and
d
-amphetamine-induced locomotion.Vertical bars represent the mean ± SEM photocell beam breaks for each group during habituation sessions (Hab) and after systemic injections of
d
-amphetamine (saline, 0.5, 1.5, 5.0 mg/kg).
Similar articles
- Effects of intra-amygdala R(+) 7-OH-DPAT on intra-accumbens d-amphetamine-associated learning. II. Instrumental conditioning.
Hitchcott PK, Phillips GD. Hitchcott PK, et al. Psychopharmacology (Berl). 1998 Dec;140(3):310-8. doi: 10.1007/s002130050772. Psychopharmacology (Berl). 1998. PMID: 9877011 - Mesoaccumbens dopamine-opiate interactions in the control over behaviour by a conditioned reinforcer.
Phillips GD, Robbins TW, Everitt BJ. Phillips GD, et al. Psychopharmacology (Berl). 1994 Mar;114(2):345-59. doi: 10.1007/BF02244858. Psychopharmacology (Berl). 1994. PMID: 7838928 - Facilitation of appetitive pavlovian conditioning by d-amphetamine in the shell, but not the core, of the nucleus accumbens.
Phillips GD, Setzu E, Hitchcott PK. Phillips GD, et al. Behav Neurosci. 2003 Aug;117(4):675-84. doi: 10.1037/0735-7044.117.4.675. Behav Neurosci. 2003. PMID: 12931953
Cited by
- The ventral striatum contributes to the activity of the motor cortex and motor outputs in monkeys.
Suzuki M, Nishimura Y. Suzuki M, et al. Front Syst Neurosci. 2022 Aug 29;16:979272. doi: 10.3389/fnsys.2022.979272. eCollection 2022. Front Syst Neurosci. 2022. PMID: 36211590 Free PMC article. - Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective.
Alcaro A, Huber R, Panksepp J. Alcaro A, et al. Brain Res Rev. 2007 Dec;56(2):283-321. doi: 10.1016/j.brainresrev.2007.07.014. Epub 2007 Aug 21. Brain Res Rev. 2007. PMID: 17905440 Free PMC article. Review. - Involvement of the dorsal striatum in cue-controlled cocaine seeking.
Vanderschuren LJ, Di Ciano P, Everitt BJ. Vanderschuren LJ, et al. J Neurosci. 2005 Sep 21;25(38):8665-70. doi: 10.1523/JNEUROSCI.0925-05.2005. J Neurosci. 2005. PMID: 16177034 Free PMC article. - At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation.
Shiflett MW, Balleine BW. Shiflett MW, et al. Eur J Neurosci. 2010 Nov;32(10):1735-43. doi: 10.1111/j.1460-9568.2010.07439.x. Epub 2010 Oct 7. Eur J Neurosci. 2010. PMID: 21044174 Free PMC article. - Dissociable hippocampal and amygdalar D1-like receptor contribution to discriminated Pavlovian conditioned approach learning.
Andrzejewski ME, Ryals C. Andrzejewski ME, et al. Behav Brain Res. 2016 Feb 15;299:111-21. doi: 10.1016/j.bbr.2015.11.034. Epub 2015 Nov 26. Behav Brain Res. 2016. PMID: 26632336 Free PMC article.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
- Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system, Vol 1, forebrain and midbrain, Ed 2. Academic; Sydney: 1995. pp. 495–578.
- Balleine B, Killcross S. Effects of ibotenic acid lesions of the nucleus accumbens on instrumental action. Behav Brain Res. 1994;65:181–193. - PubMed
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol. 1990;299:187–228. - PubMed
- Berendse HW, Galisdegraaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources