Distribution of cholinergic contacts on Renshaw cells in the rat spinal cord: a light microscopic study - PubMed (original) (raw)
Distribution of cholinergic contacts on Renshaw cells in the rat spinal cord: a light microscopic study
F J Alvarez et al. J Physiol. 1999.
Abstract
1. Cholinergic terminals in the rat spinal cord were revealed by immunohistochemical detection of the vesicular acetycholine transporter (VAChT). In order to determine the relationships of these terminals to Renshaw cells, we used dual immunolabelling with antibodies against gephyrin or calbindin D28k to provide immunohistochemical identification of Renshaw cells in lamina VII of the ventral horn. 2. A total of 50 Renshaw cells were analysed quantitatively using a computer-aided reconstruction system to provide accurate localization of contact sites and determination of somatic and dendritic surface area. Dendrites could be traced for up to 413 microm from the soma in calbindin D28k-identified Renshaw cells and up to 184 microm in gephyrin-identified cells. 3. A total of 3330 cholinergic terminals were observed on 50 Renshaw cells, with a range of 21-138 terminal appositions per cell (mean 66.6 +/- 25.56 contacts per cell). The vast majority (83.5 %) of the terminals were apposed to dendrites rather than the soma. The overall density of cholinergic contacts increased from a little above 1 per 100 microm2 on the soma and initial 25 microm of proximal dendrites to 4-5 per 100 microm2 on the surface of dendritic segments located 50-250 microm from the soma. Single presynaptic fibres frequently formed multiple contacts with the soma and/or dendrites of individual Renshaw cells. 4. VAChT-immunoreactive terminals apposed to Renshaw cells varied in size from 0.6 to 6.9 microm in diameter (mean 2.26 +/- 0.94; n = 986) and were on average smaller than the cholinergic C-terminals apposed to motoneurones, but larger than VAChT-immunoreactive terminals contacting other ventral horn interneurones. 5. The high density and relatively large size of many cholinergic terminals on Renshaw cells presumably correlates with the strong synaptic connection between motoneurones and Renshaw cells. The fact that the majority of contacts are distributed over the dendrites makes the motoneurone axon collateral input susceptible to inhibition by the prominent glycinergic inhibitory synapses located on the soma and proximal dendrites. The relative positions and structural features of the excitatory cholinergic and inhibitory glycinergic synapses may explain why Renshaw cells, although capable of firing at very high frequency following motor axon stimulation, appear to fire at relatively low rates during locomotor activity.
Figures
Figure 1. Distribution of VAChT-IR terminals and calbindin D28k-IR cells in the ventral horn of the rat spinal cord
A, low power image of the ventral horn of the rat spinal cord L5 segment immunostained with antibodies against VAChT (black-brown reaction deposits; silver-intensified DAB) and calbindin D28k (blue-black SG reaction deposits). B and C show the regions enclosed in A at higher magnification and visualized using DIC optics. VAChT immunoreactivity (brown-black) is present inside the cell bodies of presumed motoneurones (arrowheads) and in axon terminals throughout the grey matter of the ventral horn. The density of VAChT immunoreactivity inside cell bodies is relatively low, in contrast to the more intense VAChT immunoreactivity inside the terminals; the latter structures thus contain a more prominent (black) silver deposit after intensification. Small VAChT-IR terminals are present at low density throughout the grey matter of the ventral horn. Larger VAChT-IR terminals form two distinct types of arrangements in the ventral horn. The most prominent VAChT-IR terminals (corresponding to C-terminals) surround the cell bodies and proximal dendrites of motoneurones. In B, a motoneurone (brown cytoplasm) appears covered by VAChT-IR C-terminals (black). The final group of VAChT-IR terminals in the ventral horn form a plexus in ventral lamina VII. In this region, VAChT-IR terminals are predominantly associated with calbindin D28k-IR dendrites (blue-black). This area is shown at higher magnification in C. Calbindin D28k-IR cells in this area have been shown to correspond with Renshaw cells according to immunocytochemical and electrophysiological criteria (Alvarez et al. 1997; Carr et al. 1998). Other, larger, ventral horn interneurones located more dorsally also express calbindin D28k immunoreactivity (see B, blue-black neurone) but receive very little input from VAChT-IR terminals. These calbindin D28k-IR neurones were found not to display Renshaw cell characteristics (Carr et al. 1998). Scale bars are 500 μm in A and 100 μm in C. B is at the same magnification as C.
Figure 2. High magnification images showing examples of VAChT-IR terminals contacting calbindin D28k-IR Renshaw cells
A, a Renshaw cell identified according to its prominent gephyrin immunoreactivity (brown clusters covering the somatodendritic membrane) and contacted by VAChT-IR terminals (blue-black SG reaction product, arrows). B, a Renshaw cell identified by its calbindin D28k-immunoreactivity (blue-black SG reaction product) contacted by VAChT-IR terminals (black reaction product; silver-intensified DAB; arrows). C, a Renshaw cell identified by its calbindin D28k immunoreactivity (brown reaction product, DAB) contacted by VAChT-IR terminals (black reaction product; silver-intensified DAB; arrows). In all three examples, although there are some juxtasomatic contacts (arrowheads in C), most VAChT-IR contacts are located on the dendrites. The inset in C shows the diversity in sizes of VAChT-IR terminals in contact with Renshaw cell calbindin D28k-IR dendrites. A and B are at the same magnification. Scale bars in B and C, 50 μm. Scale bar in inset, 5 μm.
Figure 3. High magnification reconstruction of axon terminal collaterals displaying multiple VAChT-IR boutons (black) in contact with the somatodendritic membrane of a Renshaw cell (brown)
A series of optical sections was obtained under DIC optics and the various optical planes were superimposed to render a reconstruction of several Renshaw cell dendrites and their complement of VAChT-IR contacts. It is frequently possible to follow single VAChT-IR collaterals making multiple en passent contacts with a segment of dendrite (arrows). Scale bar, 50 μm.
Figure 4. Camera lucida reconstructions of 4 Renshaw cells from the sample identified by their calbindin D28k immunoreactivity
VAChT-IR terminals in contact with the Renshaw cells are indicated by the irregular black spots. Intervaricose portions of the presynaptic axons were omitted for clarity. VAChT-IR terminals are distributed over the whole extent of the stained dendrites, especially at distances beyond the most proximal regions (< 25 μm) of each dendrite. Scale bar, 50 μm.
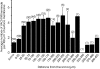
Figure 5. Histogram of the average density of VAChT-IR contacts at different distances from the cell soma
Numbers in parentheses indicate the number of Renshaw cells that displayed dendritic segments at each distance (total number of cells = 50). We were able to follow dendrites beyond a distance of 200 μm from the cell body in less than half of the Renshaw cells. Error bars indicate
s.e.m.
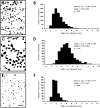
Figure 6. Size distribution of VAChT-IR terminals in relation to Renshaw cells (A and B), motoneurones (C and D) or other calbindin D28k-IR cells in the dorsal aspects of LVII (E and F)
A, C and E show examples of camera lucida drawings of each terminal population. Histograms B, D and F show the distribution of the maximun diameter of individual terminals. Scale bar in E applies to panels A and C, 10 μm.
Figure 7. Confocal images of a dual colour immunofluorescence preparation showing VAChT-IR terminals (green, FITC) and gephyrin-IR clusters (red, TRITC)
VAChT-IR terminals (arrows) were never associated with postsynaptic gephyrin clusters. The neurone illustrated (A, 15 focal planes; B, 4 focal planes) was identified as a Renshaw cell based on the size and density of the complement of gephyrin-IR clusters in the soma and proximal dendrites. Scale bar, 10 μm.
Similar articles
- Distribution of 5-hydroxytryptamine-immunoreactive boutons on immunohistochemically-identified Renshaw cells in cat and rat lumbar spinal cord.
Carr PA, Pearson JC, Fyffe RE. Carr PA, et al. Brain Res. 1999 Mar 27;823(1-2):198-201. doi: 10.1016/s0006-8993(98)01210-4. Brain Res. 1999. PMID: 10095027 - Calbindin D28k expression in immunohistochemically identified Renshaw cells.
Carr PA, Alvarez FJ, Leman EA, Fyffe RE. Carr PA, et al. Neuroreport. 1998 Aug 3;9(11):2657-61. doi: 10.1097/00001756-199808030-00043. Neuroreport. 1998. PMID: 9721951 - Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus.
Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Towart LA, et al. J Comp Neurol. 2003 Sep 1;463(4):390-401. doi: 10.1002/cne.10753. J Comp Neurol. 2003. PMID: 12836175 - Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats.
Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Alvarez FJ, et al. J Comp Neurol. 1998 Mar 30;393(1):69-83. J Comp Neurol. 1998. PMID: 9520102 - Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter.
Garzón M, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Garzón M, et al. J Comp Neurol. 1999 Jul 26;410(2):197-210. doi: 10.1002/(sici)1096-9861(19990726)410:2<197::aid-cne3>3.0.co;2-d. J Comp Neurol. 1999. PMID: 10414527 Review.
Cited by
- Postnatal phenotype and localization of spinal cord V1 derived interneurons.
Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Alvarez FJ, et al. J Comp Neurol. 2005 Dec 12;493(2):177-92. doi: 10.1002/cne.20711. J Comp Neurol. 2005. PMID: 16255029 Free PMC article. - Balanced cholinergic modulation of spinal locomotor circuits via M2 and M3 muscarinic receptors.
Nascimento F, Spindler LRB, Miles GB. Nascimento F, et al. Sci Rep. 2019 Oct 1;9(1):14051. doi: 10.1038/s41598-019-50452-1. Sci Rep. 2019. PMID: 31575899 Free PMC article. - Target selection of proprioceptive and motor axon synapses on neonatal V1-derived Ia inhibitory interneurons and Renshaw cells.
Siembab VC, Smith CA, Zagoraiou L, Berrocal MC, Mentis GZ, Alvarez FJ. Siembab VC, et al. J Comp Neurol. 2010 Dec 1;518(23):4675-701. doi: 10.1002/cne.22441. J Comp Neurol. 2010. PMID: 20963823 Free PMC article. - Identification of multiple subsets of ventral interneurons and differential distribution along the rostrocaudal axis of the developing spinal cord.
Francius C, Harris A, Rucchin V, Hendricks TJ, Stam FJ, Barber M, Kurek D, Grosveld FG, Pierani A, Goulding M, Clotman F. Francius C, et al. PLoS One. 2013 Aug 15;8(8):e70325. doi: 10.1371/journal.pone.0070325. eCollection 2013. PLoS One. 2013. PMID: 23967072 Free PMC article. - Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development.
Sapir T, Geiman EJ, Wang Z, Velasquez T, Mitsui S, Yoshihara Y, Frank E, Alvarez FJ, Goulding M. Sapir T, et al. J Neurosci. 2004 Feb 4;24(5):1255-64. doi: 10.1523/JNEUROSCI.3187-03.2004. J Neurosci. 2004. PMID: 14762144 Free PMC article.
References
- Alvarez FJ, Pearson JC, Harrington DA, Dewey DE, Torbeck L, Fyffe REW. Distribution of 5-hydroxytryptamine-immunoreactive boutons on α-motoneurons in the lumbar spinal cord of adult cats. Journal of Comparative Neurology. 1998;393:69–83. <10.1002/%28SICI%291096-9861%2819980330%29393:1<69::AID-CNE7>3.3.CO;2-X>. - DOI - PubMed
- Arvidsson U, Reidl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: A novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. Journal of Comparative Neurology. 1997;378:454–467. <10.1002/%28SICI%291096-9861%2819970224%29378:4<454::AID-CNE2>3.3.CO;2-5>. - DOI - PubMed
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York, Oxford: American Physiological Society; 1996. pp. 3–53.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources