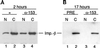The nucleoporin nup153 plays a critical role in multiple types of nuclear export - PubMed (original) (raw)
The nucleoporin nup153 plays a critical role in multiple types of nuclear export
K S Ullman et al. Mol Biol Cell. 1999 Mar.
Abstract
The fundamental process of nucleocytoplasmic transport takes place through the nuclear pore. Peripheral pore structures are presumably poised to interact with transport receptors and their cargo as these receptor complexes first encounter the pore. One such peripheral structure likely to play an important role in nuclear export is the basket structure located on the nuclear side of the pore. At present, Nup153 is the only nucleoporin known to localize to the surface of this basket, suggesting that Nup153 is potentially one of the first pore components an RNA or protein encounters during export. In this study, anti-Nup153 antibodies were used to probe the role of Nup153 in nuclear export in Xenopus oocytes. We found that Nup153 antibodies block three major classes of RNA export, that of snRNA, mRNA, and 5S rRNA. Nup153 antibodies also block the NES protein export pathway, specifically the export of the HIV Rev protein, as well as Rev-dependent RNA export. Not all export was blocked; Nup153 antibodies did not impede the export of tRNA or the recycling of importin beta to the cytoplasm. The specific antibodies used here also did not affect nuclear import, whether mediated by importin alpha/beta or by transportin. Overall, the results indicate that Nup153 is crucial to multiple classes of RNA and protein export, being involved at a vital juncture point in their export pathways. This juncture point appears to be one that is bypassed by tRNA during its export. We asked whether a physical interaction between RNA and Nup153 could be observed, using homoribopolymers as sequence-independent probes for interaction. Nup153, unlike four other nucleoporins including Nup98, associated strongly with poly(G) and significantly with poly(U). Thus, Nup153 is unique among the nucleoporins tested in its ability to interact with RNA and must do so either directly or indirectly through an adaptor protein. These results suggest a unique mechanistic role for Nup153 in the export of multiple cargos.
Figures
Figure 1
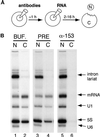
Antibodies to Nup153 prevent the export of multiple classes of RNA. (A) The oocyte injection protocol is shown. First, antibodies were injected into the nucleus. After a 60 min incubation, a second nuclear injection was performed, delivering radioactively labeled RNA. The oocytes were incubated for periods of varying duration and then manually dissected. Nuclear (N) and cytoplasmic (C) RNA was prepared and analyzed by gel electrophoresis. (B) After initial injections either with buffer (BUF., lanes 1 and 2), preimmune IgG (PRE, lanes 3 and 4), or affinity-purified antibodies to Nup153 (α-153, lanes 5 and 6), oocytes were next injected with a mixture of RNA (pre-mRNA, U1 snRNA, 5S rRNA, and U6 snRNA). Two hours later, nuclear and cytoplasmic RNA was harvested. The pre-mRNA has been processed into mature mRNA and a lariat intron. The intron, as well as U6 snRNA, is not normally exported; their nuclear localization here confirms the accuracy of the injection and dissection. Nuclear export of mRNA, U1 snRNA, and 5S RNA is substantially underway at this 2 h time point (lanes 2 and 4) but is fully prevented in the presence of anti-Nup153 antibodies (lane 6).
Figure 2
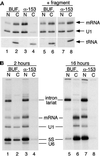
Antibodies to Nup153 have a long-lasting and specific inhibitory effect on mRNA export but fail to block tRNA export. (A) An injection experiment was performed as described in Figure 1, except that, in some cases, a recombinant fragment of Nup153 was coinjected with the first injection of buffer or antibodies (lanes 5–8). The mix of RNAs in this experiment included tRNA in addition to pre-mRNA and U1 snRNA. Antibodies to Nup153 did not prevent tRNA export (compare lanes 2 and 4), whereas, as before, export of mRNA and U1 snRNA was blocked by these antibodies. When the Nup153 fragment was coinjected with anti-Nup153 antibodies (lanes 7 and 8), the effect of the antibody was neutralized (lane 8). (B) After injection of either buffer or antibodies, oocytes were injected with a mixture of pre-mRNA, U1 snRNA, 5S RNA, and U6 snRNA and then harvested after either 2 h (lanes 1–4) or 16 h (lanes 5–8) of incubation at 18°C. U6 snRNA is relatively less stable and is no longer detected at the later time point. Export of mRNA, U1 snRNA, and 5S RNA was inhibited at both early and late time points in oocytes preinjected with antibodies to Nup153.
Figure 3
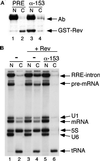
Nup153 is required for Rev protein export and Rev-mediated RNA export. (A) After injection with either preimmune IgG (lanes 1 and 2) or antibodies to Nup153 (lanes 3 and 4), a recombinant form of the HIV protein Rev (GST–Rev) was injected into the oocyte nuclei. After 2 h, protein was harvested from dissected oocytes and analyzed by Western blot. Rev protein export was inhibited by anti-Nup153 antibodies (compare lanes 2 and 4). The preimmune and affinity-purified antibodies injected into the oocyte nuclei are detected by the secondary antibody (goat anti-rabbit IgG) used in the Western blot (lanes 1 and 3, Ab). (B) Control and antibody-injected oocytes were injected with a mixture of pre-mRNA, U1 snRNA, 5S RNA, U6 snRNA, and tRNA. The pre-mRNA used here contains a Rev response element (RRE) in the intron. Where indicated, GST–Rev was also included in the RNA mixture (lanes 3–6). In the presence of GST–Rev, the RRE-containing lariat intron as well as the pre-mRNA was exported (compare lanes 2 and 4). However, antibodies against Nup153 block Rev-mediated RRE export (lane 6).
Figure 4
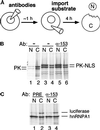
Nuclear import occurs in the presence of antibodies to Nup153. (A) The oocyte injection protocol is shown. Antibodies were injected into the nucleus, and after a 60 min incubation, radioactively labeled import substrates were injected into the cytoplasm. After 4 h of incubation, oocytes were manually dissected, and nuclear and cytoplasmic protein was prepared and analyzed by gel electrophoresis. (B) After cytoplasmic injection, pyruvate kinase (PK) remains primarily cytoplasmic (lanes 1 and 2). In contrast, a substantial amount of an NLS-containing form of PK migrates to the nucleus after cytoplasmic injection (lanes 3 and 4). This NLS-directed import continues in the presence of anti-Nup153 antibodies (lanes 5 and 6). (C) M9-directed import of hnRNPA1 also took place in the presence of antibodies to Nup153 (lanes 3 and 4 vs. lanes 1 and 2). Luciferase, coinjected into the cytoplasm with hnRNPA1, remained primarily cytoplasmic, serving as a control for the specificity of nuclear accumulation.
Figure 5
Importin β exports in the presence of anti-Nup153 antibodies. (A) After either no treatment or antibody injection, recombinant 6XHis-importin β (Imp.-β) was injected into the oocyte nuclei. The localization of His-tagged importin β was then examined at an early time point (2 h) when its export was still underway. The nucleocytoplasmic distribution of 6XHis-importin β was similar in oocytes that were either untreated (lanes 1 and 2) or injected with anti-Nup153 antibodies (lanes 3 and 4) before the injection of importin β. This indicates that the kinetics of importin β recycling are unaltered by the presence of antibodies to Nup153. (B) In an independent experiment, recombinant importin β was injected into nuclei after injection of either preimmune IgG or anti-Nup153 antibodies. Protein was harvested from nuclear and cytoplasmic fractions 17 h later. His-tagged importin β equilibrated to the cytoplasm even in the presence of Nup153-specific antibodies (lanes 2 and 4).
Figure 6
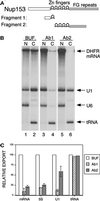
Antibodies to distinct regions of Nup153 affect export differentially. (A) The domain structure of Xenopus Nup153 is represented schematically, showing the unique N-terminal region, the five zinc fingers, and the FG-containing C-terminal region. Antibody 1 (Ab1) was raised against a fragment containing part of the N-terminal region and one zinc finger (amino acids 53–334). Antibody 2 (Ab2) was raised against the remaining four zinc fingers and part of the FG region (amino acids 334–828). (B) Oocytes were injected as described in Figure 1, first with buffer or antibodies and then with radiolabeled RNA. The RNA mixture used here contained DHFR mRNA (an intronless mRNA), U1 snRNA, U6 snRNA, and tRNA. RNA localization was analyzed after a 4 h incubation. DHFR mRNA export was inhibited equally by Ab1 and Ab2, whereas U1 RNA export is significantly less sensitive to Ab2 (compare lanes 2, 4, and 6). (C) The effects of Ab1 and Ab2 are depicted in this bar chart in which data from multiple experiments are summarized. Inhibitory profiles of antibodies to distinct regions of Nup153 are shown here for the adenoviral mRNA (spliced), U1 snRNA, 5S rRNA, and tRNA.
Figure 7
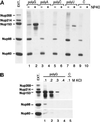
Nup153 interacts, directly or indirectly, with poly(G) and poly(U). (A) Affinity resins bearing the homoribopolymer indicated (lanes 1–8) or resin alone (lanes 9 and 10; control [C]) were incubated with Xenopus egg extract in the absence (lanes 1, 3, 5, 7, and 9) or presence (lanes 2, 4, 6, 8, and 10) of 0.5% NP-40. After the beads were washed (see MATERIALS AND METHODS), proteins that remained associated were eluted in SDS sample buffer and analyzed by Western blot. mAb414 and antibodies specific for Nup98 were used to detect a panel of nucleoporins. Nup358, Nup214, Nup153, Nup98, and Nup60 are all detected in egg extract (Ext. [left], an amount equivalent to 28% of the material included in each incubation is loaded). Some nonspecific binding of Nup98 and Nup60 is found in the absence of NP-40 when the nucleoporins recovered with the control beads are examined (lane 9). Levels over this background binding are clearly detected for Nup153 with poly(G) (lane 1), and this binding is greatly augmented by the presence of NP-40 (lane 2). Nup153 also associated with poly(U) in the presence of NP-40 (lane 8). (B) A binding experiment similar to that described above was performed with a matrix bearing the poly(G) ribopolymer and an identical control matrix lacking ribopolymer. Nucleoporin recovery was examined in the presence of increasing amounts of KCl and with NP-40 (0.2%) present. Nup153 remained associated with poly(G) when the KCl concentration was as high as 300 mM (lane 3). The left panel of egg extract represents 16% of the loaded material.
Similar articles
- Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr.
Shah S, Tugendreich S, Forbes D. Shah S, et al. J Cell Biol. 1998 Apr 6;141(1):31-49. doi: 10.1083/jcb.141.1.31. J Cell Biol. 1998. PMID: 9531546 Free PMC article. - Novel vertebrate nucleoporins Nup133 and Nup160 play a role in mRNA export.
Vasu S, Shah S, Orjalo A, Park M, Fischer WH, Forbes DJ. Vasu S, et al. J Cell Biol. 2001 Oct 29;155(3):339-54. doi: 10.1083/jcb.200108007. Epub 2001 Oct 29. J Cell Biol. 2001. PMID: 11684705 Free PMC article. - Separate nuclear import pathways converge on the nucleoporin Nup153 and can be dissected with dominant-negative inhibitors.
Shah S, Forbes DJ. Shah S, et al. Curr Biol. 1998 Dec 17-31;8(25):1376-86. doi: 10.1016/s0960-9822(98)00018-9. Curr Biol. 1998. PMID: 9889100 - Versatility at the nuclear pore complex: lessons learned from the nucleoporin Nup153.
Ball JR, Ullman KS. Ball JR, et al. Chromosoma. 2005 Nov;114(5):319-30. doi: 10.1007/s00412-005-0019-3. Epub 2005 Nov 12. Chromosoma. 2005. PMID: 16133350 Review. - Review: transport of tRNA out of the nucleus-direct channeling to the ribosome?
Grosshans H, Simos G, Hurt E. Grosshans H, et al. J Struct Biol. 2000 Apr;129(2-3):288-94. doi: 10.1006/jsbi.2000.4226. J Struct Biol. 2000. PMID: 10806079 Review.
Cited by
- Localization of nucleoporin Tpr to the nuclear pore complex is essential for Tpr mediated regulation of the export of unspliced RNA.
Rajanala K, Nandicoori VK. Rajanala K, et al. PLoS One. 2012;7(1):e29921. doi: 10.1371/journal.pone.0029921. Epub 2012 Jan 13. PLoS One. 2012. PMID: 22253824 Free PMC article. - Nuclear routing networks span between nuclear pore complexes and genomic DNA to guide nucleoplasmic trafficking of biomolecules.
Malecki M, Malecki B. Malecki M, et al. J Fertili In Vitro. 2012 Oct 19;2(4):112. doi: 10.4172/2165-7491.1000112. J Fertili In Vitro. 2012. PMID: 23275893 Free PMC article. - Nucleoporins in cardiovascular disease.
Burdine RD, Preston CC, Leonard RJ, Bradley TA, Faustino RS. Burdine RD, et al. J Mol Cell Cardiol. 2020 Apr;141:43-52. doi: 10.1016/j.yjmcc.2020.02.010. Epub 2020 Mar 21. J Mol Cell Cardiol. 2020. PMID: 32209327 Free PMC article. Review. - Control of mammalian gene expression by selective mRNA export.
Wickramasinghe VO, Laskey RA. Wickramasinghe VO, et al. Nat Rev Mol Cell Biol. 2015 Jul;16(7):431-42. doi: 10.1038/nrm4010. Epub 2015 Jun 17. Nat Rev Mol Cell Biol. 2015. PMID: 26081607 Review. - Human nucleoporins promote HIV-1 docking at the nuclear pore, nuclear import and integration.
Di Nunzio F, Danckaert A, Fricke T, Perez P, Fernandez J, Perret E, Roux P, Shorte S, Charneau P, Diaz-Griffero F, Arhel NJ. Di Nunzio F, et al. PLoS One. 2012;7(9):e46037. doi: 10.1371/journal.pone.0046037. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23049930 Free PMC article.
References
- Adam SA, Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–847. - PubMed
- Aitchison JD, Blobel G, Rout MP. Kap104p: a karyopherin involved in the nuclear transport of mRNA binding proteins. Science. 1996;274:624–627. - PubMed
- Arts GJ, Fornerod M, Mattaj IW. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources