Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha - PubMed (original) (raw)
Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha
A Y Yu et al. J Clin Invest. 1999 Mar.
Abstract
Chronic hypoxia induces polycythemia, pulmonary hypertension, right ventricular hypertrophy, and weight loss. Hypoxia-inducible factor 1 (HIF-1) activates transcription of genes encoding proteins that mediate adaptive responses to hypoxia, including erythropoietin, vascular endothelial growth factor, and glycolytic enzymes. Expression of the HIF-1alpha subunit increases exponentially as O2 concentration is decreased. Hif1a-/- mouse embryos with complete deficiency of HIF-1alpha due to homozygosity for a null allele at the Hif1a locus die at midgestation, with multiple cardiovascular malformations and mesenchymal cell death. Hif1a+/- heterozygotes develop normally and are indistinguishable from Hif1a+/+ wild-type littermates when maintained under normoxic conditions. In this study, the physiological responses of Hif1a+/- and Hif1a+/+ mice exposed to 10% O2 for one to six weeks were analyzed. Hif1a+/- mice demonstrated significantly delayed development of polycythemia, right ventricular hypertrophy, pulmonary hypertension, and pulmonary vascular remodeling and significantly greater weight loss compared with wild-type littermates. These results indicate that partial HIF-1alpha deficiency has significant effects on multiple systemic responses to chronic hypoxia.
Figures
Figure 1
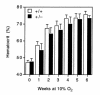
Development of polycythemia in mice subjected to chronic hypoxia. Hematocrits of Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice exposed to room air or 10% O2 for 1–6 weeks were determined. Results are expressed as mean ± SE (n = 8–10 mice for 0–5 weeks; n = 5–7 mice for 6 weeks). ANOVA with a post hoc Dunnet's test revealed a significant difference between genotypes (P = 0.025).
Figure 2
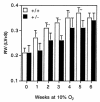
Development of right ventricular hypertrophy in response to chronic hypoxia. The mass ratio of the right ventricle (RV) to left ventricle and septum (LV+S) was determined for the same Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice analyzed in Fig. 1. Results are expressed as mean ± SE. ANOVA with a post hoc Dunnet's test revealed a significant difference between genotypes (P = 0.0001). *P < 0.01 ; **P < 0.001 (Student's t test).
Figure 3
Measurement of right ventricular pressures. Shown are representative polygraph tracings obtained from Hif1a+/+ and Hif1a+/– mice exposed to room air (Normoxic) or 10% O2 (Hypoxic) for 3 weeks.
Figure 4
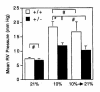
Right ventricular pressures of normoxic, hypoxic, and reoxygenated mice. Mean right ventricular (RV) pressure (± SE) was determined for Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice exposed to 21% (n = 6) or 10% (n = 11–15) O for 3 weeks, or exposed to 10% O for 3 weeks followed by 21% O for 3 h (n = 5). *P = 0.003; #P = NS (Student's t test). NS, not significant.
Figure 5
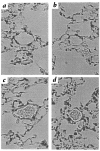
Pulmonary histology. Lungs from Hif1a+/+ (a and c) and Hif1a+/+ (b and d) mice exposed to 21% (a and b) or 10% (c and d) O for 3 weeks were formalin-fixed, paraffin-embedded, sectioned, and stained with hematoxylin and eosin for videomicroscopy. Each field shows a representative pulmonary arteriole. ×400.
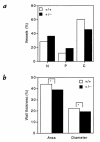
Figure 6
Morphometric analysis of pulmonary vasculature in chronically hypoxic mice. (a) Neomuscularization of pulmonary arterioles. Lung sections from Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice exposed to 10% O for 3 weeks were scored for nonmuscularized (N), partially muscularized (P), or completely muscularized (C) arterioles with an external diameter of ≤100 μm. For each genotype, >400 vessels were analyzed in lung sections from three to four mice to generate the mean data shown. χ2 analysis revealed a significant difference between genotypes (P = 0.00001). (b) Quantitative analysis of medial thickening. Percent wall thickness (% WT) was calculated for completely muscularized arterioles, based on analysis of area or diameter, according to the following formulae: % WT = ([areaext – areaint] / areaext) × 100; and % WT = ([diameterext – diameterint] / diameterext) × 100. Dimensions were demarcated by the external (ext) and internal (int) elastic laminae. For each genotype, >100 vessels were analyzed in multiple lung sections from three to four mice. Mean values are shown (SD ≤ 0.6% for each). *P < 0.001 (Student's t test).
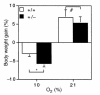
Figure 7
Analysis of weight gain under normoxic and hypoxic conditions. Percent body weight gain (% BW gain) was determined for Hif1a+/+ (open bars) and Hif1a+/– (closed bars) mice exposed to 21% O for 6 weeks (n = 10) or 10% O for 1–6 weeks (n = 54–57), using the following formula: % BW gai_n_ = ([BWfinal – BWinitial] / BWinitial) × 100. *P = 0.02; #P = NS (Student's t test).
Similar articles
- Partial HIF-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia.
Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Shimoda LA, et al. Am J Physiol Lung Cell Mol Physiol. 2001 Jul;281(1):L202-8. doi: 10.1152/ajplung.2001.281.1.L202. Am J Physiol Lung Cell Mol Physiol. 2001. PMID: 11404263 - Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death.
Kotch LE, Iyer NV, Laughner E, Semenza GL. Kotch LE, et al. Dev Biol. 1999 May 15;209(2):254-67. doi: 10.1006/dbio.1999.9253. Dev Biol. 1999. PMID: 10328919 - Gene expression profiling of sex differences in HIF1-dependent adaptive cardiac responses to chronic hypoxia.
Bohuslavová R, Kolář F, Kuthanová L, Neckář J, Tichopád A, Pavlinkova G. Bohuslavová R, et al. J Appl Physiol (1985). 2010 Oct;109(4):1195-202. doi: 10.1152/japplphysiol.00366.2010. Epub 2010 Jul 15. J Appl Physiol (1985). 2010. PMID: 20634361 - O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1.
Semenza GL. Semenza GL. J Appl Physiol (1985). 2004 Mar;96(3):1173-7; discussion 1170-2. doi: 10.1152/japplphysiol.00770.2003. J Appl Physiol (1985). 2004. PMID: 14766767 Review. - Hypoxia regulation of gene transcription.
Caro J. Caro J. High Alt Med Biol. 2001 Summer;2(2):145-54. doi: 10.1089/152702901750265251. High Alt Med Biol. 2001. PMID: 11442996 Review.
Cited by
- Distinct responses to hypoxia in subpopulations of distal pulmonary artery cells contribute to pulmonary vascular remodeling in emphysema.
Howard LS, Crosby A, Vaughan P, Sobolewski A, Southwood M, Foster ML, Chilvers ER, Morrell NW. Howard LS, et al. Pulm Circ. 2012 Apr-Jun;2(2):241-9. doi: 10.4103/2045-8932.97616. Pulm Circ. 2012. PMID: 22837865 Free PMC article. - Loss of microRNA-17∼92 in smooth muscle cells attenuates experimental pulmonary hypertension via induction of PDZ and LIM domain 5.
Chen T, Zhou G, Zhou Q, Tang H, Ibe JC, Cheng H, Gou D, Chen J, Yuan JX, Raj JU. Chen T, et al. Am J Respir Crit Care Med. 2015 Mar 15;191(6):678-92. doi: 10.1164/rccm.201405-0941OC. Am J Respir Crit Care Med. 2015. PMID: 25647182 Free PMC article. - Clinical iron deficiency disturbs normal human responses to hypoxia.
Frise MC, Cheng HY, Nickol AH, Curtis MK, Pollard KA, Roberts DJ, Ratcliffe PJ, Dorrington KL, Robbins PA. Frise MC, et al. J Clin Invest. 2016 Jun 1;126(6):2139-50. doi: 10.1172/JCI85715. Epub 2016 May 3. J Clin Invest. 2016. PMID: 27140401 Free PMC article. - Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period.
Carreras A, Kayali F, Zhang J, Hirotsu C, Wang Y, Gozal D. Carreras A, et al. Am J Physiol Regul Integr Comp Physiol. 2012 Oct 1;303(7):R700-9. doi: 10.1152/ajpregu.00258.2012. Epub 2012 Aug 15. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22895743 Free PMC article. - Role of RhoB in the regulation of pulmonary endothelial and smooth muscle cell responses to hypoxia.
Wojciak-Stothard B, Zhao L, Oliver E, Dubois O, Wu Y, Kardassis D, Vasilaki E, Huang M, Mitchell JA, Harrington LS, Prendergast GC, Wilkins MR. Wojciak-Stothard B, et al. Circ Res. 2012 May 25;110(11):1423-34. doi: 10.1161/CIRCRESAHA.112.264473. Epub 2012 Apr 26. Circ Res. 2012. PMID: 22539766 Free PMC article.
References
- Hultgren HN, Grover RF. Circulatory adaptation to high altitude. Annu Rev Med. 1968;19:119–152. - PubMed
- Naeije R. Pulmonary circulation at high altitude. Respiration. 1997;64:429–434. - PubMed
- DiCarlo VS, et al. ETA-receptor antagonism prevents and reverses chronic hypoxia-induced pulmonary hypertension in rat. Am J Physiol. 1995;269:L690–L697. - PubMed
- Hales CA, Kradin RL, Brandstetter RD, Zhu Y-J. Impairment of hypoxic pulmonary artery remodeling by heparin in mice. Am Rev Respir Dis. 1983;128:747–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL55338/HL/NHLBI NIH HHS/United States
- R01 HL055338/HL/NHLBI NIH HHS/United States
- R01-DK39869/DK/NIDDK NIH HHS/United States
- R01 HL051912/HL/NHLBI NIH HHS/United States
- R01-HL51912/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases