HLA class I deficiencies due to mutations in subunit 1 of the peptide transporter TAP1 - PubMed (original) (raw)
HLA class I deficiencies due to mutations in subunit 1 of the peptide transporter TAP1
H de la Salle et al. J Clin Invest. 1999 Mar.
Abstract
The transporter associated with antigen processing (TAP), which is composed of two subunits (TAP1 and TAP2) that have different biochemical and functional properties, plays a key role in peptide loading and the cell surface expression of HLA class I molecules. Three cases of HLA class I deficiency have previously been shown to result from the absence of a functional TAP2 subunit. In the present study, we analyzed two cases displaying not only the typical lung syndrome of HLA class I deficiency but also skin lesions, and found these patients to be TAP1-deficient. This defect leads to unstable HLA class I molecules and their retention in the endoplasmic reticulum. However, the absence of TAP1 is compatible with life and does not seem to result in higher susceptibility to viral infections than TAP2 deficiency. This work also reveals that vasculitis is often observed in HLA class I-deficient patients.
Figures
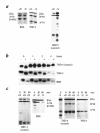
Figure 1
Absence of maturation and instability of HLA class I molecules in HLA class I–deficient patients. (a) BRE and TND-3 cells from HLA class I–deficient patients and MRC-5 HLA class I+ control cells were metabolically labeled, lysed, and HLA class I molecules immunoprecipitated with W6/32 (pan anti–HLA class I heavy chains). Immunoprecipitates were treated (+N) or not (–N) with neuraminidase and analyzed by IEF. (b) BRE and TND-3 (HLA class I–) and TND-4 (HLA class I+) cells were metabolically labeled for 20 min and chased for 0, 1, 2, or 4 hours. HLA class I molecules were immunoprecipitated with W6/32, treated (+) or not (–) with Endo H, and separated by SDS PAGE. (c) Cells were metabolically labeled for 30 min and chased for 30 min, and HLA class I molecules were immunoprecipitated with B1G6 (anti-β2m) and adsorbed on protein–agarose beads. The adsorbed proteins were further incubated for 0, 30, or 60 min at 37°C in lysis buffer, and the beads were washed. After treatment (+N) or not (–N) with neuraminidase, the immunoprecipitated proteins were eluted and analyzed by IEF. IEF, isoelectric focusing gel electrophoresis; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; β2m, β2-microglobulin.
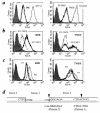
Figure 2
BRE and TND-3 cell lines are TAP1 deficient. (a) Cells were labeled with the MAB W6/32 and analyzed by flow cytometry. Closed histograms, isotype control. Thick lines: left, BRE fibroblast cell line from patient 1; right, TND-3 EBV-B cell line from patient 2. Dotted lines: left, STF-1 TAP2-deficient fibroblast cell line; right, ST-EMO TAP2-deficient EBV-B cell line. Thin lines: left, STF-6 normal fibroblast cell line; right, ST-EAH normal EBV-B cell line. (b) Cells were infected overnight with TAP1 or TAP2 recombinant vaccinia viruses, labeled with W6/32, and analyzed by flow cytometry. Left, BRE cells from patient 1; right, TND-3 cells from patient 2. (c) BRE and TND-3 cells were incubated overnight at 26°C in culture medium (RPMI 1640 supplemented with 10% FCS; Life Technologies) in the presence of 100 μg/ml synthetic peptides, then labeled with MAB W6/32 and analyzed by flow cytometry. Closed histograms, isotype control. Thick lines, incubation without peptide. Thin lines: P1, HLA-A2402-specific peptide; P2, HLA-A2601-specific peptide. (d) Molecular genetic characterization of the mutation. Upper sequence, normal TAP1 sequence; lower sequences, corresponding TAP1 sequences in patients 1 and 2. G indicates the exon 2 nucleotide involved in the splicing reaction between exon 1 and exon 2, and arrows point to the positions of the two mutations.
Similar articles
- Restoration of the expression of transports associated with antigen processing in human malignant melanoma increases tumor-specific immunity.
Tao J, Li Y, Liu YQ, Wang L, Yang J, Dong J, Wu Y, Shen GX, Tu YT. Tao J, et al. J Invest Dermatol. 2008 Aug;128(8):1991-6. doi: 10.1038/jid.2008.10. Epub 2008 Apr 3. J Invest Dermatol. 2008. PMID: 18385764 - Identification of domain boundaries within the N-termini of TAP1 and TAP2 and their importance in tapasin binding and tapasin-mediated increase in peptide loading of MHC class I.
Procko E, Raghuraman G, Wiley DC, Raghavan M, Gaudet R. Procko E, et al. Immunol Cell Biol. 2005 Oct;83(5):475-82. doi: 10.1111/j.1440-1711.2005.01354.x. Immunol Cell Biol. 2005. PMID: 16174096 - TAP1 and TAP2 polymorphisms and their linkage disequilibrium with HLA-DR, -DP, and -DQ in an eastern Andalusian population.
Alvarado-Guerri R, Cabrera CM, Garrido F, López-Nevot MA. Alvarado-Guerri R, et al. Hum Immunol. 2005 Aug;66(8):921-30. doi: 10.1016/j.humimm.2005.06.009. Epub 2005 Aug 16. Hum Immunol. 2005. PMID: 16216677 - Clinical and immunological aspects of HLA class I deficiency.
Zimmer J, Andrès E, Donato L, Hanau D, Hentges F, de la Salle H. Zimmer J, et al. QJM. 2005 Oct;98(10):719-27. doi: 10.1093/qjmed/hci112. Epub 2005 Aug 8. QJM. 2005. PMID: 16087697 Review. - A novel mutation in TAP1 gene leading to MHC class I deficiency: Report of two cases and review of the literature.
Hanalioglu D, Ayvaz DC, Ozgur TT, van der Burg M, Sanal O, Tezcan I. Hanalioglu D, et al. Clin Immunol. 2017 May;178:74-78. doi: 10.1016/j.clim.2017.01.011. Epub 2017 Feb 2. Clin Immunol. 2017. PMID: 28161407 Review.
Cited by
- Viral inhibition of the transporter associated with antigen processing (TAP): a striking example of functional convergent evolution.
Verweij MC, Horst D, Griffin BD, Luteijn RD, Davison AJ, Ressing ME, Wiertz EJ. Verweij MC, et al. PLoS Pathog. 2015 Apr 16;11(4):e1004743. doi: 10.1371/journal.ppat.1004743. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25880312 Free PMC article. Review. - Targeted degradation of ABC transporters in health and disease.
Nikles D, Tampé R. Nikles D, et al. J Bioenerg Biomembr. 2007 Dec;39(5-6):489-97. doi: 10.1007/s10863-007-9120-z. J Bioenerg Biomembr. 2007. PMID: 17972020 Review. - Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells.
Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, Dromer C, Emilie D, Moreau JF, Déchanet-Merville J. Halary F, et al. J Exp Med. 2005 May 16;201(10):1567-78. doi: 10.1084/jem.20041851. J Exp Med. 2005. PMID: 15897274 Free PMC article. - Transporter associated with antigen processing 1 (TAP1) expression and prognostic analysis in breast, lung, liver, and ovarian cancer.
Tabassum A, Samdani MN, Dhali TC, Alam R, Ahammad F, Samad A, Karpiński TM. Tabassum A, et al. J Mol Med (Berl). 2021 Sep;99(9):1293-1309. doi: 10.1007/s00109-021-02088-w. Epub 2021 May 28. J Mol Med (Berl). 2021. PMID: 34047812 Free PMC article. - Molecular studies and NK cell function of a new case of TAP2 homozygous human deficiency.
Matamoros N, Milà J, Llano M, Balas A, Vicario JL, Pons J, Crespí C, Martinez N, Iglesias-Alzueta J, López-Botet M. Matamoros N, et al. Clin Exp Immunol. 2001 Aug;125(2):274-82. doi: 10.1046/j.1365-2249.2001.01595.x. Clin Exp Immunol. 2001. PMID: 11529920 Free PMC article.
References
- de la Salle, H., et al. 1998. HLA class I deficiencies. In Primary immunodeficiency diseases: a molecular and genetic approach. H.D. Ochs, C.E.I. Smith, and J.M. Puck, editors. Oxford University Press. New York, NY. 181–188.
- Watanabe S, Iwata M, Maeda H, Ishibashi Y. Immunohistochemical studies of major histocompatibility antigens in a case of the bare lymphocyte syndrome without immunodeficiency. J Am Acad Dermatol. 1987;17:895–900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous