Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9-A resolution - PubMed (original) (raw)
Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9-A resolution
V N Malashkevich et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Ebola virions contain a surface transmembrane glycoprotein (GP) that is responsible for binding to target cells and subsequent fusion of the viral and host-cell membranes. GP is expressed as a single-chain precursor that is posttranslationally processed into the disulfide-linked fragments GP1 and GP2. The GP2 subunit is thought to mediate membrane fusion. A soluble fragment of the GP2 ectodomain, lacking the fusion-peptide region and the transmembrane helix, folds into a stable, highly helical structure in aqueous solution. Limited proteolysis studies identify a stable core of the GP2 ectodomain. This 74-residue core, denoted Ebo-74, was crystallized, and its x-ray structure was determined at 1.9-A resolution. Ebo-74 forms a trimer in which a long, central three-stranded coiled coil is surrounded by shorter C-terminal helices that are packed in an antiparallel orientation into hydrophobic grooves on the surface of the coiled coil. Our results confirm the previously anticipated structural similarity between the Ebola GP2 ectodomain and the core of the transmembrane subunit from oncogenic retroviruses. The Ebo-74 structure likely represents the fusion-active conformation of the protein, and its overall architecture resembles several other viral membrane-fusion proteins, including those from HIV and influenza.
Figures
Figure 1
A schematic representation of Ebola GP. The residues are numbered according to their position in the Ebola GP sequence (Zaire subtype; Genbank accession no. U23187). The sequence of Ebo-74 is indicated. Ebola GP is proteolytically processed into the receptor binding domain GP1 (residues 33–501; gray), and the transmembrane domain GP2 (residues 502–676; light blue). Close to the N terminus, GP2 contains the fusion peptide (FP, residues 524–539). Two regions predicted to form amphipatic helical regions are indicated (blue and red). GP2 is presumed to be disulfide-linked to GP1 through Cys-609. The C-terminal part of the first helical region and most of the loop correspond to an immunosupressive motif in oncogenic retroviruses (residues 584–610; black stripe). The transmembrane anchor (TM, residues 651–672) attaches GP2 to the viral membrane. Identical residues between the sequences of Ebo-74 and Mo-55 from Mo-MLV (10, 12) are indicated with lines, and similar residues are indicated with dots. The chloride-binding residue, Asn-586, is marked by ∗. SS; signal sequence.
Figure 2
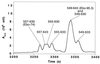
Protein dissection of the Ebola GP2 ectodomain. Shown is a portion of a reverse-phase HPLC chromatogram of a sample of Ebo-95.3 digested with protease K for 2 hr in 20 mM Tris, pH 8.7. The HPLC eluant was analyzed directly with an on-line mass spectrometer (Finnigan-MAT LCQ), and the fragments (indicated with residue numbers) were assigned by matching observed masses with a list of all possible predicted fragment masses (see text). Fragments corresponding to peptides described in this paper are labeled with parentheses.
Figure 3
Ebo-74 forms a trimer-of-hairpins structure. (A) A side view of the Ebo-74 structure. N helices (dark blue) constitute a central coiled coil and C helices (red) pack into hydrophobic grooves on the surface of the coiled coil. Disulfides within the loop regions (light blue) are depicted in yellow. (B) Each monomer has an α-helical hairpin conformation. The last three turns of the N helix, followed by the short α- and 310-helices (magenta), correspond to the immunosuppressive motif region in oncogenic retroviruses. Figure was generated by using
insight ii
(64).
Figure 4
Chloride-binding site within the central coiled coil of Ebo-74 as visualized in a stereo view of the final 2_F_o − _F_c electron density map. A chloride ion and water molecules are shown as green and red spheres, respectively. The map was contoured at 1.5 σ. Figure was generated by using o (26).
Figure 5
Remarkable similarity between filovirus and retrovirus membrane-fusion proteins. A stereo view of the superposition of the Ebo-74 (blue) and the Mo-55 (yellow) structures is shown. The location of the chloride ion is almost identical in the superposition. The largest deviations occur at the N termini and in the loop regions of the Ebo-74 and Mo-55 structures. The rms deviation values for the Cα atoms of Ebo-74 and Mo-55 monomers (and values for trimers in parentheses) are as follows: entire structure 1.56 Å (1.84 Å); coiled-coil cores 0.49 Å (0.94 Å); loop regions 1.91 Å (2.33 Å). Figure was generated by using
insight ii
(64).
Figure 6
Trimer-of-hairpins structures in viral membrane-fusion proteins. Side and top views of the fusion proteins from three different groups of viruses are shown. (Top) Ebola (this work and ref. 17) and Mo-MLV (10). (Middle) SIV (16, 33) and HIV-1 (14, 31, 32). (Bottom) Influenza (41). The N-terminal coiled-coil cores are depicted in dark blue. In all structures, the trimer-of-hairpins conformation brings the N and C termini, and therefore the fusion peptides and transmembrane helices, into close proximity. Figure was generated by using
insight ii
(64).
Similar articles
- Crystal structure of the Marburg virus GP2 core domain in its postfusion conformation.
Koellhoffer JF, Malashkevich VN, Harrison JS, Toro R, Bhosle RC, Chandran K, Almo SC, Lai JR. Koellhoffer JF, et al. Biochemistry. 2012 Oct 2;51(39):7665-75. doi: 10.1021/bi300976m. Epub 2012 Sep 19. Biochemistry. 2012. PMID: 22935026 Free PMC article. - Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain.
Weissenhorn W, Carfí A, Lee KH, Skehel JJ, Wiley DC. Weissenhorn W, et al. Mol Cell. 1998 Nov;2(5):605-16. doi: 10.1016/s1097-2765(00)80159-8. Mol Cell. 1998. PMID: 9844633 - Functional importance of the coiled-coil of the Ebola virus glycoprotein.
Watanabe S, Takada A, Watanabe T, Ito H, Kida H, Kawaoka Y. Watanabe S, et al. J Virol. 2000 Nov;74(21):10194-201. doi: 10.1128/jvi.74.21.10194-10201.2000. J Virol. 2000. PMID: 11024148 Free PMC article. - The central structural feature of the membrane fusion protein subunit from the Ebola virus glycoprotein is a long triple-stranded coiled coil.
Weissenhorn W, Calder LJ, Wharton SA, Skehel JJ, Wiley DC. Weissenhorn W, et al. Proc Natl Acad Sci U S A. 1998 May 26;95(11):6032-6. doi: 10.1073/pnas.95.11.6032. Proc Natl Acad Sci U S A. 1998. PMID: 9600912 Free PMC article. - Marburg virus glycoprotein GP2: pH-dependent stability of the ectodomain α-helical bundle.
Harrison JS, Koellhoffer JF, Chandran K, Lai JR. Harrison JS, et al. Biochemistry. 2012 Mar 27;51(12):2515-25. doi: 10.1021/bi3000353. Epub 2012 Mar 12. Biochemistry. 2012. PMID: 22369502 Free PMC article.
Cited by
- The Role of the Charged Residues of the GP2 Helical Regions in Ebola Entry().
Jiang H, Wang J, Manicassamy B, Manicassamy S, Caffrey M, Rong L. Jiang H, et al. Virol Sin. 2009 Apr;24(2):121-135. doi: 10.1007/s12250-009-3015-6. Virol Sin. 2009. PMID: 23227032 Free PMC article. - Structural and Functional Studies on the Marburg Virus GP2 Fusion Loop.
Liu N, Tao Y, Brenowitz MD, Girvin ME, Lai JR. Liu N, et al. J Infect Dis. 2015 Oct 1;212 Suppl 2(Suppl 2):S146-53. doi: 10.1093/infdis/jiv030. Epub 2015 Mar 18. J Infect Dis. 2015. PMID: 25786917 Free PMC article. - Crystal structure of the Marburg virus GP2 core domain in its postfusion conformation.
Koellhoffer JF, Malashkevich VN, Harrison JS, Toro R, Bhosle RC, Chandran K, Almo SC, Lai JR. Koellhoffer JF, et al. Biochemistry. 2012 Oct 2;51(39):7665-75. doi: 10.1021/bi300976m. Epub 2012 Sep 19. Biochemistry. 2012. PMID: 22935026 Free PMC article. - N- and C-terminal residues combine in the fusion-pH influenza hemagglutinin HA(2) subunit to form an N cap that terminates the triple-stranded coiled coil.
Chen J, Skehel JJ, Wiley DC. Chen J, et al. Proc Natl Acad Sci U S A. 1999 Aug 3;96(16):8967-72. doi: 10.1073/pnas.96.16.8967. Proc Natl Acad Sci U S A. 1999. PMID: 10430879 Free PMC article. - LearnCoil-VMF: computational evidence for coiled-coil-like motifs in many viral membrane-fusion proteins.
Singh M, Berger B, Kim PS. Singh M, et al. J Mol Biol. 1999 Jul 30;290(5):1031-41. doi: 10.1006/jmbi.1999.2796. J Mol Biol. 1999. PMID: 10438601 Free PMC article.
References
- Jahring, P. B., Kiley, M. P., Klenk, H.-D., Peters, C. J., Sanchez, A. & Swanepoel, R. (1995) Arch. Virol.10, Suppl., 289–292.
- Centers for Disease Control and Prevention. Morbid Mortal Week Rep. 1995;44:381–382. - PubMed
- Feldmann H, Klenk H-D. Adv Virus Res. 1996;47:1–52. - PubMed
- Yang Z, Delgado R, Xu L, Todd R F, Nabel E G, Sanchez A, Nabel G J. Science. 1998;279:1034–1037. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical