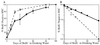In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells - PubMed (original) (raw)
In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells
S H Cheshier et al. Proc Natl Acad Sci U S A. 1999.
Abstract
A rare set of hematopoietic stem cells (HSC) must undergo a massive expansion to produce mature blood cells. The phenotypic isolation of HSC from mice offers the opportunity to determine directly their proliferation kinetics. We analyzed the proliferation and cell cycle kinetics of long-term self-renewing HSC (LT-HSC) in normal adult mice. At any one time, approximately 5% of LT-HSC were in S/G2/M phases of the cell cycle and another 20% were in G1 phase. BrdUrd incorporation was used to determine the rate at which different cohorts of HSC entered the cell cycle over time. About 50% of LT-HSC incorporated BrdUrd by 6 days and >90% incorporated BrdUrd by 30 days. By 6 months, 99% of LT-HSC had incorporated BrdUrd. We calculated that approximately 8% of LT-HSC asynchronously entered the cell cycle per day. Nested reverse transcription-PCR analysis revealed cyclin D2 expression in a high proportion of LT-HSC. Although approximately 75% of LT-HSC are quiescent in G0 at any one time, all HSC are recruited into cycle regularly such that 99% of LT-HSC divide on average every 57 days.
Figures
Figure 1
Double-sorting is necessary to obtain pure KTSL−/lo and KTSL− cells. (Upper) c-kit (_y_-axis) and lineage (_x_-axis) density plots with outliers. Boxes indicate sort gates for KTSL−/lo (inner and outer boxes) and KTSL− (inner boxes only). (Lower) Sca-1 (_y_-axis) and Thy 1.1 (_x_-axis) density plots with outliers. Boxes indicate sort gates for KTSL−/lo and KTSL− cells. As seen in the second and third columns, significant numbers of cells outside the sort gates are present in a reanalysis of a single sorted sample. After the samples are double-sorted, the great majority of cells (≥95%) are within the sort gates that define KTSL−/lo (fourth column) and KTSL− (fifth column) HSC.
Figure 2
In vivo BrdUrd incorporation kinetics of KTSL−/lo and KTSL− cells. (A) BrdUrd incorporation rate of KTSL−/lo cells (□) and KTSL− cells (●). Each data point represents mean percentages of BrdUrd positive HSC from at least two separate experiments with a combined analysis of at least 500 cells. Some error bars are smaller than their associated symbol. (B) BrdUrd incorporation means plotted in semilogarithmic fashion as the proportion of BrdUrd-negative KTSL−/lo cells (□) and KTSL− cells (●) against time in days. The lines were generated by using least squares fit linear regression.
Figure 3
Cyclin D2 expression in five (A) and one (B) KTSL− cells as determined by using nested RT-PCR. No RT indicates lanes with RT-PCR reactions performed on KTSL− without the initial addition of reverse transcriptase to the RT reactions as a control.
Figure 4
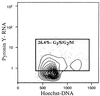
Growth Fraction of KTSL− LT-HSC. FACS analysis of double-sorted KTSL− cells stained with PY (y axis) and Hoechst 33342 (x axis). The growth fraction of a population of cells is the fraction of actively dividing cells (G1/S/G2/M). On average, 23.5 ± 3.4% of KTSL− LT-HSC were in G1/S/G2/M at any one time.
Figure 5
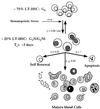
Model of LT-HSC cell cycle regulation. LT-HSC are asynchronously dividing with a constant fraction of cells in the cell cycle and a constant fraction in G0. LT-HSC are continuously moving into and out of the cell cycle at rates indicated next to the arrows (p denotes probability of labeled event occurring) as determined from the BrdUrd incorporation kinetics of KTSL− cells. _T_c = apparent cell cycle time.
Similar articles
- Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle.
Wright DE, Cheshier SH, Wagers AJ, Randall TD, Christensen JL, Weissman IL. Wright DE, et al. Blood. 2001 Apr 15;97(8):2278-85. doi: 10.1182/blood.v97.8.2278. Blood. 2001. PMID: 11290588 - GATA-3 regulates hematopoietic stem cell maintenance and cell-cycle entry.
Ku CJ, Hosoya T, Maillard I, Engel JD. Ku CJ, et al. Blood. 2012 Mar 8;119(10):2242-51. doi: 10.1182/blood-2011-07-366070. Epub 2012 Jan 20. Blood. 2012. PMID: 22267605 Free PMC article. - Adaptation to ex vivo culture reduces human hematopoietic stem cell activity independently of the cell cycle.
Johnson CS, Williams M, Sham K, Belluschi S, Ma W, Wang X, Lau WWY, Kaufmann KB, Krivdova G, Calderbank EF, Mende N, McLeod J, Mantica G, Li J, Grey-Wilson C, Drakopoulos M, Basheer S, Sinha S, Diamanti E, Basford C, Wilson NK, Howe SJ, Dick JE, Göttgens B, Green AR, Francis N, Laurenti E. Johnson CS, et al. Blood. 2024 Aug 15;144(7):729-741. doi: 10.1182/blood.2023021426. Blood. 2024. PMID: 38805639 Free PMC article. - Beyond "to divide or not to divide": Kinetics matters in hematopoietic stem cells.
Johnson C, Belluschi S, Laurenti E. Johnson C, et al. Exp Hematol. 2020 Dec;92:1-10.e2. doi: 10.1016/j.exphem.2020.11.003. Epub 2020 Nov 11. Exp Hematol. 2020. PMID: 33188890 Review. - In vivo divisional tracking of hematopoietic stem cells.
Takizawa H, Manz MG. Takizawa H, et al. Ann N Y Acad Sci. 2012 Aug;1266:40-6. doi: 10.1111/j.1749-6632.2012.06500.x. Ann N Y Acad Sci. 2012. PMID: 22901254 Review.
Cited by
- Mouse multipotent progenitor 5 cells are located at the interphase between hematopoietic stem and progenitor cells.
Sommerkamp P, Romero-Mulero MC, Narr A, Ladel L, Hustin L, Schönberger K, Renders S, Altamura S, Zeisberger P, Jäcklein K, Klimmeck D, Rodriguez-Fraticelli A, Camargo FD, Perié L, Trumpp A, Cabezas-Wallscheid N. Sommerkamp P, et al. Blood. 2021 Jun 10;137(23):3218-3224. doi: 10.1182/blood.2020007876. Blood. 2021. PMID: 33754628 Free PMC article. - Redox Control in Acute Lymphoblastic Leukemia: From Physiology to Pathology and Therapeutic Opportunities.
Chen Y, Li J, Zhao Z. Chen Y, et al. Cells. 2021 May 17;10(5):1218. doi: 10.3390/cells10051218. Cells. 2021. PMID: 34067520 Free PMC article. Review. - Biomechanical Regulation of Hematopoietic Stem Cells in the Developing Embryo.
Horton PD, Dumbali SP, Bhanu KR, Diaz MF, Wenzel PL. Horton PD, et al. Curr Tissue Microenviron Rep. 2021 Mar;2(1):1-15. doi: 10.1007/s43152-020-00027-4. Epub 2021 Jan 26. Curr Tissue Microenviron Rep. 2021. PMID: 33937868 Free PMC article. - Mutation accumulation and developmental lineages in normal and Down syndrome human fetal haematopoiesis.
Hasaart KAL, Manders F, van der Hoorn ML, Verheul M, Poplonski T, Kuijk E, de Sousa Lopes SMC, van Boxtel R. Hasaart KAL, et al. Sci Rep. 2020 Jul 31;10(1):12991. doi: 10.1038/s41598-020-69822-1. Sci Rep. 2020. PMID: 32737409 Free PMC article. - Single-cell transcriptome analyses reveal critical regulators of spermatogonial stem cell fate transitions.
Li S, Yan RG, Gao X, He Z, Wu SX, Wang YJ, Zhang YW, Tao HP, Zhang XN, Jia GX, Yang QE. Li S, et al. BMC Genomics. 2024 Feb 3;25(1):138. doi: 10.1186/s12864-024-10072-0. BMC Genomics. 2024. PMID: 38310206 Free PMC article.
References
- Metcalf D. The Molecular Control of Blood Cells. Cambridge, MA: Harvard Univ. Press; 1988.
- McCulloch E A, Till J E. Radiat Res. 1960;13:115. - PubMed
- Morrison S J, Weissman I L. Immunity. 1994;1:661–673. - PubMed
- Kay H E M. Lancet. 1965;ii:418–419. - PubMed
- Lemischka I R, Raulet D H, Mulligan R C. Cell. 1986;45:917–927. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous