The role of immunophilins in mutant superoxide dismutase-1linked familial amyotrophic lateral sclerosis - PubMed (original) (raw)
The role of immunophilins in mutant superoxide dismutase-1linked familial amyotrophic lateral sclerosis
J P Lee et al. Proc Natl Acad Sci U S A. 1999.
Abstract
It has been reported that expression of familial amyotrophic lateral sclerosis (FALS)-associated mutant Cu/Zn superoxide dismutase-1 (SOD) induces apoptosis of neuronal cells in culture associated with an increase in reactive oxygen species. SOD recently has been shown to prevent calcineurin inactivation, initiating the present investigations examining the role of calcineurin in mutant SOD-induced cell death. Wild-type or mutant SOD was expressed in neuronal cells by infection with replication-deficient adenoviruses. PC12 cells overexpressing human wild-type SOD exhibited higher calcineurin activity than cells expressing FALS-related mutant SOD (SODV148G); however, cells expressing SODV148G had calcineurin activity equal to mock-infected cells, suggesting that cell death induced by mutant SOD was not related to a decrease in calcineurin activity. Calcineurin antagonists such as cyclosporin A and FK506, as well as nonimmunosuppressant analogs of cyclosporin A, significantly enhanced SODV148G- and SODA4V-induced cell death. Because both groups of drugs inhibit the rotamase activity of cyclophilins (CyP), but only the immunosuppressant analogs inhibit calcineurin activity, these data suggest that rotamase inhibition underlies the enhanced cell death after SODV148G expression. The importance of rotamase activity in mutant SOD-mediated apoptosis was supported by experiments showing that overexpressed wild-type cyclophilin A (CyPA), but not CyPA with a rotamase active site point mutation, protected cells from death after SODV148G expression. These data suggest that mutant SOD produces a greater need for rotamase and, also, highlights possible new therapeutic strategies in FALS.
Figures
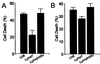
Figure 1
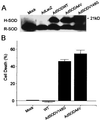
(A) Western blot analysis of SOD from PC12 cells 3 days after mock infection (lane 1) or infection with a control AdLacZ (lane 2), AdSODWT (lane 3), AdSODA4V (lane 4), or AdSODV148G (lane 5). Cell lysates were subjected to SDS/PAGE, were blotted onto nitrocellulose, and then were incubated with an anti-SOD polyclonal antibody. Note the immunoreactivity in all lanes of a protein corresponding to the electrophoretic mobility of the rodent form of SOD (R-SOD) at ≈19 kDa (lower band). In the case of infection with AdSODWT, AdSODA4V, and AdSODV148G (lanes 3–5, respectively), an immunostained protein at ≈21 kDa (upper band) is seen, corresponding to the electrophoretic mobility of human SOD (H-SOD). (B) Cell death of differentiated PC12 cells 5 days after infection with mock, AdSODWT AdSODV148G, or AdSODA4V. Expression of SODV148G and SODA4V caused cell death whereas there was no significant alteration in the viability of control cells or SODWT-expressing cells.
Figure 2
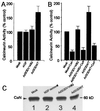
Assessment of CsA influence on calcineurin activity and immunoblot analysis of calcineurin at a time before evidence of apoptotic cell loss. (A) Calcineurin activity was assayed 3 days after AdV infection or 1 day after NGF withdrawal. Cells expressing SODWT exhibited higher calcineurin activity than mock-infected cells, cells expressing SODV148G, or after NGF withdrawal. (B) Treatment with CsA (1 μM) dramatically decreased calcineurin activity in all cases. (C) Similar immunostaining of calcineurin is seen in Western blot analysis after mock (lane 1), NGF removal (lane 2), SODV148G (lane 3), or SODWT expression (lane 4).
Figure 3
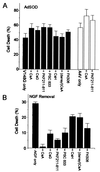
CsA and its immunosuppressant and nonimmunosuppressant analogs potentiate cell death induced by SODV148G (black bars) and SODA4V expression (open bars) in PC12 cells (A) and show a protective effect after NGF removal (B). Drugs were added immediately after virus infection and were replenished in fresh media every 3 days. CsA, CsG, and FK506 are immunosuppressive drugs that inhibit calcineurin. PKF 211–811 and PSC833 are nonimmunosuppressants that do not inhibit calcineurin but do inhibit rotamase activity. The cell death induced by FALS-related SODV148G was potentiated by both drug classes (as was SODA4V) but not by CsH and
d
-lys(dansyl)-8-Cs. Values are expressed as means ± SEM (n = 3).
Figure 4
The effect of the immunosuppressant CsA or its nonimmunosuppressive analog PKF211–811 on cell survival of hippocampal neurons 6 days after infection with AdSODV148G (A) and after treatment with NMDA (100 μM) (B). Treatment with these drugs did not significantly change the viability of mock-infected cells or cells expressing SODWT (data not shown). Values are expressed as means ± SEM (n = 3).
Figure 5
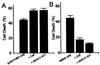
Effect of overexpression of WTCyPA or an isomerase-deficient CyPA(R55A) on PC12 cell death induced by SODV148G expression (A) or after NGF withdrawal (B). As a control, cells were transfected with CD8 cDNA. Values are expressed as means ± SEM (n = 3).
Similar articles
- No correlation between aggregates of Cu/Zn superoxide dismutase and cell death in familial amyotrophic lateral sclerosis.
Lee JP, Gerin C, Bindokas VP, Miller R, Ghadge G, Roos RP. Lee JP, et al. J Neurochem. 2002 Sep;82(5):1229-38. doi: 10.1046/j.1471-4159.2002.01056.x. J Neurochem. 2002. PMID: 12358770 - Mutant superoxide dismutase-1-linked familial amyotrophic lateral sclerosis: molecular mechanisms of neuronal death and protection.
Ghadge GD, Lee JP, Bindokas VP, Jordan J, Ma L, Miller RJ, Roos RP. Ghadge GD, et al. J Neurosci. 1997 Nov 15;17(22):8756-66. doi: 10.1523/JNEUROSCI.17-22-08756.1997. J Neurosci. 1997. PMID: 9348345 Free PMC article. - Superoxide dismutase mutations of familial amyotrophic lateral sclerosis and the oxidative inactivation of calcineurin.
Volkel H, Scholz M, Link J, Selzle M, Werner P, Tunnemann R, Jung G, Ludolph AC, Reuter A. Volkel H, et al. FEBS Lett. 2001 Aug 17;503(2-3):201-5. doi: 10.1016/s0014-5793(01)02730-2. FEBS Lett. 2001. PMID: 11513882 - [Familial amyotrophic lateral sclerosis and mutations in the Cu/Zn superoxide dismutase gene].
Nakano R. Nakano R. Rinsho Shinkeigaku. 1995 Dec;35(12):1546-8. Rinsho Shinkeigaku. 1995. PMID: 8752459 Review. Japanese. - Superoxide dismutase and the death of motoneurons in ALS.
Beckman JS, Estévez AG, Crow JP, Barbeito L. Beckman JS, et al. Trends Neurosci. 2001 Nov;24(11 Suppl):S15-20. doi: 10.1016/s0166-2236(00)01981-0. Trends Neurosci. 2001. PMID: 11881740 Review.
Cited by
- The involvement of mammalian and plant FK506-binding proteins (FKBPs) in development.
Breiman A, Camus I. Breiman A, et al. Transgenic Res. 2002 Aug;11(4):321-35. doi: 10.1023/a:1016331814412. Transgenic Res. 2002. PMID: 12212836 Review. - Proteomics reveals novel oxidative and glycolytic mechanisms in type 1 diabetic patients' skin which are normalized by kidney-pancreas transplantation.
Folli F, Guzzi V, Perego L, Coletta DK, Finzi G, Placidi C, La Rosa S, Capella C, Socci C, Lauro D, Tripathy D, Jenkinson C, Paroni R, Orsenigo E, Cighetti G, Gregorini L, Staudacher C, Secchi A, Bachi A, Brownlee M, Fiorina P. Folli F, et al. PLoS One. 2010 Mar 29;5(3):e9923. doi: 10.1371/journal.pone.0009923. PLoS One. 2010. PMID: 20360867 Free PMC article. - Targeting Extracellular Cyclophilin A Reduces Neuroinflammation and Extends Survival in a Mouse Model of Amyotrophic Lateral Sclerosis.
Pasetto L, Pozzi S, Castelnovo M, Basso M, Estevez AG, Fumagalli S, De Simoni MG, Castellaneta V, Bigini P, Restelli E, Chiesa R, Trojsi F, Monsurrò MR, Callea L, Malešević M, Fischer G, Freschi M, Tortarolo M, Bendotti C, Bonetto V. Pasetto L, et al. J Neurosci. 2017 Feb 8;37(6):1413-1427. doi: 10.1523/JNEUROSCI.2462-16.2016. Epub 2016 Dec 23. J Neurosci. 2017. PMID: 28011744 Free PMC article. - Synaptic sprouting increases the uptake capacities of motoneurons in amyotrophic lateral sclerosis mice.
Millecamps S, Nicolle D, Ceballos-Picot I, Mallet J, Barkats M. Millecamps S, et al. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7582-7. doi: 10.1073/pnas.131031098. Epub 2001 Jun 12. Proc Natl Acad Sci U S A. 2001. PMID: 11404466 Free PMC article. - Cyclophilin A: a key player for human disease.
Nigro P, Pompilio G, Capogrossi MC. Nigro P, et al. Cell Death Dis. 2013 Oct 31;4(10):e888. doi: 10.1038/cddis.2013.410. Cell Death Dis. 2013. PMID: 24176846 Free PMC article. Review.
References
- Rosen D R, Siddique T, Patterson D, Figlewicz D A, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan J P, Deng H X, et al. Nature (London) 1993;364:59–62. - PubMed
- McCord J M, Fridovich I. J Biol Chem. 1969;244:6049–6055. - PubMed
- Gurney M E, Pu H, Chiu A Y, Dal Canto M C, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon Y W, Deng H X, et al. Science. 1994;264:1772–1775. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical