Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo - PubMed (original) (raw)
Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo
A M Babic et al. Mol Cell Biol. 1999 Apr.
Abstract
Fisp12 was first identified as a secreted protein encoded by a growth factor-inducible immediate-early gene in mouse fibroblasts, whereas its human ortholog, CTGF (connective tissue growth factor), was identified as a mitogenic activity in conditioned media of human umbilical vein endothelial cells. Fisp12/CTGF is a member of a family of secreted proteins that includes CYR61, Nov, Elm-1, Cop-1/WISP-2, and WISP-3. Fisp12/CTGF has been shown to promote cell adhesion and mitogenesis in both fibroblasts and endothelial cells and to stimulate cell migration in fibroblasts. These findings, together with the localization of Fisp12/CTGF in angiogenic tissues, as well as in atherosclerotic plaques, suggest a possible role for Fisp12/CTGF in the regulation of vessel growth during development, wound healing, and vascular disease. In this study, we show that purified Fisp12 (mCTGF) protein promotes the adhesion of microvascular endothelial cells through the integrin receptor alphavbeta3. Furthermore, Fisp12 stimulates the migration of microvascular endothelial cells in culture, also through an integrin-alphavbeta3-dependent mechanism. In addition, the presence of Fisp12 promotes endothelial cell survival when cells are plated on laminin and deprived of growth factors, a condition that otherwise induces apoptosis. In vivo, Fisp12 induces neovascularization in rat corneal micropocket implants. These results demonstrate that Fisp12 is a novel angiogenic inducer and suggest a direct role for Fisp12 in the adhesion, migration, and survival of endothelial cells during blood vessel growth. Taken together with the recent finding that the related protein CYR61 also induces angiogenesis, we suggest that Fisp12/mCTGF and CYR61 comprise prototypes of a new family of angiogenic regulators that function, at least in part, through integrin-alphavbeta3-dependent pathways.
Figures
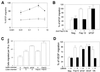
FIG. 1
Fisp12 mediates HMVEC adhesion through integrin αvβ3. HMVECs were washed and harvested in PBS with 1 mM EDTA, resuspended in serum-free medium, and plated on microtiter wells coated with indicated substrates. After incubation at 37°C for 30 min, adherent cells were fixed and stained with methylene blue, followed by quantitation by measuring the absorbance at 620 nm. Data shown are the means of duplicate determinations, and similar results were obtained in at least three separate experiments. Error bars represent the standard deviation(s) (SD). (A) Dose dependence of adhesion to Fisp12. HMVECs were plated onto microtiter wells coated with the indicated concentrations of purified Fisp12. (B) Divalent cation dependence. HMVEC adhesion to either BSA- or Fisp12-coated plates was determined; EDTA (10 mM) or Ca2+ (20 mM) was added where indicated. (C) Inhibition by RGD peptides. HMVECs were incubated with either buffer (control) or 0.2 or 2.0 mM GRGDSP or GRGESP peptides, as indicated, prior to addition to the microtiter wells coated with Fisp12 (5 μg/ml), 2 μg of fibronectin per ml (FN), or 0.1 μg of vitronectin per ml (VN). (D) HMVEC adhesion to Fisp12 was dependent on integrin αvβ3. Microtiter wells were coated with BSA, Fisp12 (2.5 μg/ml), fibronectin (2 μg/ml), or vitronectin (0.1 μg/ml). HMVECs were incubated with either normal mouse immunoglobulin G or the anti-αvβ3 antibody LM609 (50 μg/ml) prior to plating, and adhesion was measured at 30 min thereafter.
FIG. 2
Fisp12 stimulates HMVEC migration through an αvβ3-dependent pathway. The migration of HMVECs was measured in a modified Boyden chamber assay. The cells placed in a lower chamber that migrated into the upper chamber were counted in three high-power fields for each condition ± the SD after a 4-h incubation at 37°C. As chemoattractants, bFGF (10 ng/ml), VEGF (1 ng/ml), vitronectin (5 μg/ml), and Fisp12 (1 μg/ml unless otherwise indicated) was placed in either the top or the bottom chamber, or both, as indicated. (A) Fisp12-stimulated cell migration is dose dependent. Cells that migrated into the upper well where the indicated amount of purified Fisp12 or the corresponding amount of the Fisp12 storage buffer was placed, were counted. The results are expressed as the percentage of bFGF-induced migration ± the SD. (B) Specific inhibition of Fisp12-induced cell migration by anti-Fisp12 antibodies. HMVEC migration was measured as described above by using either Fisp12 or bFGF as the chemoattractant. Where indicated, these proteins were preincubated with anti-Fisp12 antibodies (30 μg/ml) before addition to the upper wells. Neg., background migration in the absence of chemoattractant. (C) Fisp12 induces chemokinesis. The migration of HMVECs was measured in a checkerboard-type analysis. Fisp12 or bFGF were added to the upper chamber, the lower chamber, neither chamber, or both chambers as indicated. (D) Specific inhibition of Fisp12-induced HMVEC migration by anti-αvβ3 antibody. HMVEC migration was monitored by using Fisp12, vitronectin, bFGF, or VEGF as the chemoattractants. Where indicated cells were preincubated with 50 μg of LM609 per ml for 1 h before addition to the lower chamber. The results are expressed as the percentage of cells that migrated to VEGF. Neg., background migration in the absence of chemoattractant.
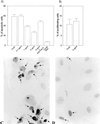
FIG. 3
Fisp12 protects HMVECs from apoptosis. HMVECs were starved for 24 h prior to the addition to slides coated with 10 μg of laminin (Collaborative biosciences) per ml overnight at 4°C. Cells were then incubated in EBM for 20 h with or without the addition of Fisp12 protein, and apoptosis was monitored by using the TUNEL assay; the number of apoptotic cells was then counted. (A) Dose dependence of Fisp12-promoted cell survival. Values for HMVECs incubated in the absence of Fisp12 (control) or in the presence of indicated concentrations of Fisp12 are shown. Where indicated, Fisp12 was preincubated with anti-Fisp12 antibody prior to addition to the medium. Complete medium was added as a positive control. The percentages of apoptotic cells ± the SD from at least 500 cells counted are shown, and each experiment was done in triplicate. (B) Cell proliferation assay. HMVECs treated as described in panel A were labeled with BrdUrd for 24 h, and the percentages of cells incorporating label in the absence or presence of Fisp12 are shown. (C and D) Photomicrographs of HMVECs incubated in the absence or presence of 5 μg of Fisp12 per ml, respectively.
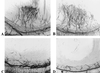
FIG. 4
Fisp12 induces neovascularization in rat corneas. Hydron pellets containing test substances were formulated and implanted into corneas of rats as described in Materials and Methods and Table 1. The formation of new blood vessels was visualized by perfusion with colloidal carbon 7 days after implantation. Hydron pellets contained in Fisp12 (A), bFGF (B), Fisp12 storage buffer (C), and Fisp12 protein preincubated with anti-Fisp12 antibodies (D) are shown.
Similar articles
- Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin alpha(IIb)beta(3).
Jedsadayanmata A, Chen CC, Kireeva ML, Lau LF, Lam SC. Jedsadayanmata A, et al. J Biol Chem. 1999 Aug 20;274(34):24321-7. doi: 10.1074/jbc.274.34.24321. J Biol Chem. 1999. PMID: 10446209 - Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells.
Leu SJ, Lam SC, Lau LF. Leu SJ, et al. J Biol Chem. 2002 Nov 29;277(48):46248-55. doi: 10.1074/jbc.M209288200. Epub 2002 Oct 2. J Biol Chem. 2002. PMID: 12364323 - CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family.
Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. Lin CG, et al. J Biol Chem. 2003 Jun 27;278(26):24200-8. doi: 10.1074/jbc.M302028200. Epub 2003 Apr 13. J Biol Chem. 2003. PMID: 12695522 - Connective tissue growth factor: what's in a name?
Moussad EE, Brigstock DR. Moussad EE, et al. Mol Genet Metab. 2000 Sep-Oct;71(1-2):276-92. doi: 10.1006/mgme.2000.3059. Mol Genet Metab. 2000. PMID: 11001822 Review. - Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts.
Grotendorst GR. Grotendorst GR. Cytokine Growth Factor Rev. 1997 Sep;8(3):171-9. doi: 10.1016/s1359-6101(97)00010-5. Cytokine Growth Factor Rev. 1997. PMID: 9462483 Review.
Cited by
- CCN2 induces cellular senescence in fibroblasts.
Jun JI, Lau LF. Jun JI, et al. J Cell Commun Signal. 2017 Mar;11(1):15-23. doi: 10.1007/s12079-016-0359-1. Epub 2016 Oct 18. J Cell Commun Signal. 2017. PMID: 27752926 Free PMC article. - Cartilage-specific over-expression of CCN family member 2/connective tissue growth factor (CCN2/CTGF) stimulates insulin-like growth factor expression and bone growth.
Tomita N, Hattori T, Itoh S, Aoyama E, Yao M, Yamashiro T, Takigawa M. Tomita N, et al. PLoS One. 2013;8(3):e59226. doi: 10.1371/journal.pone.0059226. Epub 2013 Mar 28. PLoS One. 2013. PMID: 23555635 Free PMC article. - Matricellular proteins in cardiac adaptation and disease.
Frangogiannis NG. Frangogiannis NG. Physiol Rev. 2012 Apr;92(2):635-88. doi: 10.1152/physrev.00008.2011. Physiol Rev. 2012. PMID: 22535894 Free PMC article. Review. - WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase.
Su F, Overholtzer M, Besser D, Levine AJ. Su F, et al. Genes Dev. 2002 Jan 1;16(1):46-57. doi: 10.1101/gad.942902. Genes Dev. 2002. PMID: 11782444 Free PMC article. - Role of the CCN protein family in cancer.
Kim H, Son S, Shin I. Kim H, et al. BMB Rep. 2018 Oct;51(10):486-492. doi: 10.5483/BMBRep.2018.51.10.192. BMB Rep. 2018. PMID: 30158025 Free PMC article.
References
- Bates R C, Lincz L F, Burns G F. Involvement of integrins in cell survival. Cancer Metastasis Rev. 1995;14:191–203. - PubMed
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–130. - PubMed
- Bouck N, Stellmach V, Hsu S C. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous