An activator binding module of yeast RNA polymerase II holoenzyme - PubMed (original) (raw)
An activator binding module of yeast RNA polymerase II holoenzyme
Y C Lee et al. Mol Cell Biol. 1999 Apr.
Abstract
The Mediator complex of Saccharomyces cerevisiae is required for both general and regulated transcription of RNA polymerase II (PolII) and is composed of two stable subcomplexes (Srb4 and Rgr1 subcomplexes). To decipher the function of each Mediator subcomplex and to delineate the functional relationship between the subcomplexes, we characterized the compositions and biochemical activities of PolII-Mediator complexes (holoenzymes) prepared from several Mediator mutant strains of S. cerevisiae. We found that holoenzymes devoid of a functional Gal11 module were defective for activated but not basal transcription in a reconstituted in vitro system. This activation-specific defect was correlated with a crippled physical interaction to transcriptional activator proteins, which could be bypassed by artificial recruitment of a mutant holoenzyme to a promoter. Consistent with this observation, a direct interaction between Gal11 and gene-specific transcriptional activator proteins was detected by far-Western analyses and column binding assays. In contrast, the srb5 deletion mutant holoenzyme was defective for both basal and activated transcription, despite its capacity for activator binding that is comparable to that of the wild-type holoenzyme. These results demonstrate that the Gal11 module of the Rgr1 subcomplex is required for the efficient recruitment of PolII holoenzyme to a promoter via activator-specific interactions, while the Srb4 subcomplex functions in the modulation of general polymerase activity.
Figures
FIG. 1
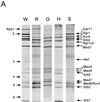
Subunit compositions of mutant holoenzymes. (A) Immunoprecipitation analysis. Holoenzymes (MonoQ fractions) were prepared from wild-type (YCL10; W), _rgr1_Δ2 (DY2010; R), _gal11_Δ (HS301; G), _hrs1_Δ (SSAB-2CF; H), and _srb5_Δ (CTY153; S) strains and immunoprecipitated with anti-Rgr1 antibody–beads as described in Materials and Methods. Proteins were resolved on an SDS–10% polyacrylamide gel and visualized by silver staining. The positions of core polymerase subunits (Rpb) and Mediator components are indicated at the left and right, respectively. (B) Immunoblot analysis. Wild-type (W) and mutant (R, _rgr1_Δ2; S, _srb5_Δ; G, _gal11_Δ; H, _hrs1_Δ) holoenzymes (MonoQ fractions) were subjected to immunoblot analysis with antisera specific for the PolII and Mediator components indicated between the panels.
FIG. 1
Subunit compositions of mutant holoenzymes. (A) Immunoprecipitation analysis. Holoenzymes (MonoQ fractions) were prepared from wild-type (YCL10; W), _rgr1_Δ2 (DY2010; R), _gal11_Δ (HS301; G), _hrs1_Δ (SSAB-2CF; H), and _srb5_Δ (CTY153; S) strains and immunoprecipitated with anti-Rgr1 antibody–beads as described in Materials and Methods. Proteins were resolved on an SDS–10% polyacrylamide gel and visualized by silver staining. The positions of core polymerase subunits (Rpb) and Mediator components are indicated at the left and right, respectively. (B) Immunoblot analysis. Wild-type (W) and mutant (R, _rgr1_Δ2; S, _srb5_Δ; G, _gal11_Δ; H, _hrs1_Δ) holoenzymes (MonoQ fractions) were subjected to immunoblot analysis with antisera specific for the PolII and Mediator components indicated between the panels.
FIG. 2
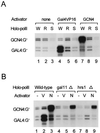
In vitro transcription of mutant holoenzymes. Wild-type and mutant holoenzymes (700 ng each) containing the same levels of nonspecific transcriptional elongation activities were analyzed for their promoter-specific transcriptional activities in the presence of the indicated activators in an in vitro transcription system reconstituted with pure general transcription factors and other supplements as described previously (21). RNA transcripts from templates containing either the Gal4 binding site (GAL4:G−) or the GCN4 binding site (GCN4:G−) are indicated. (A) Transcriptional defects of _rgr1_Δ2 and _srb5_Δ holoenzymes. The specifically initiated transcripts from reactions that contained wild-type (W), _rgr1_Δ2 (R), and _srb5_Δ (S) holoenzymes in the absence (none) or presence of activator protein (Gal4VP16 or GCN4; 30 ng each) are shown. (B) Transcriptional defects of _gal11_Δ and _hrs1_Δ holoenzymes. The specifically initiated transcripts from reactions that contained wild-type, _gal11_Δ, and _hrs1_Δ holoenzymes in the absence (−) or presence of activator protein (Gal4VP16 [V] or GCN4 [N]; 30 ng each) are shown.
FIG. 3
TFIIH-dependent CTD phosphorylation of mutant holoenzymes. (A) Core polymerase (core-polII, 0.3 μg) and holoenzyme (Holo-polII, 0.9 μg) were incubated with [γ-32P]ATP and without (−) or with (+) the indicated supplements (Gal4VP16 [Gal4VP, 30 ng], TBP [50 ng], TFIIB [50 ng], TFIIE [60 ng], TFIIH [60 ng], TFIIF [F, 20 ng], and DNA [pJJ470, 200 ng]) in transcription buffer for 10 min (holoenzyme) or 40 min (core polymerase) at 25°C. Phosphorylated Rpb1 was analyzed by SDS-PAGE (7.5% gel) and visualized by autoradiography. (B) CTD phosphorylation of mutant holoenzymes during Gal4VP16-mediated transcriptional activation. The degrees of CTD phosphorylation of the indicated holoenzymes (900 ng) under various transcription reaction conditions are shown. CTD phosphorylation by TFIIH (60 ng) only in the presence of the DNA template (pJJ470, 200 ng; lane H) and under basal (lane B) or activated (Gal4VP16 mediated, lane A) transcription conditions is shown. (C) CTD phosphorylation of mutant holoenzymes during GCN4-mediated transcriptional activation. CTD of wild-type (W), _rgr1_Δ2 (R), _srb5_Δ (S), _gal11_Δ (G), and _hrs1_Δ (H) holoenzymes was phosphorylated by TFIIH under basal or activated (GCN4-mediated) transcription conditions.
FIG. 4
Interactions of transcriptional activators with wild-type and mutant holoenzymes. (A, B, and D) Plotted is the change in the refractory index units (RU) versus time after injection of the indicated holoenzymes onto an activator-immobilized Biosensor chip. (A) SPR analysis of the interactions between Gal4VP16 and wild-type and _rgr1_Δ2 holoenzymes (70 μl of a MonoQ fraction at a concentration of 200 μg/ml). (B) SPR analysis of the interactions between wild-type (W) and _hrs1_Δ (H) holoenzymes (50 μl of a MonoQ fraction at a concentration of 250 μg/ml) and GST-VP16 or GCN4. (C) Coimmunoprecipitation (I.P.) of Gal4VP16 with wild-type (W) and mutant (R, _rgr1_Δ2) holoenzymes followed by Western blotting. Each holoenzyme (MonoQ fraction, 10 μg) was mixed with Gal4VP16 (300 ng) and immunoprecipitated with anti-Rgr1 antibody–beads (see Materials and Methods). In order to measure the amounts of precipitated Gal4VP16 and holoenzymes, we included equimolar amounts of recombinant Gal4VP16 and Med6 proteins (r-P; Med6, 80 ng; Gal4VP16, 60 ng) as quantitative standards. Immunoblot analyses with antisera specific for the proteins indicated to the right of the panel are shown. (D) SPR analysis of the interactions between wild-type (W, 150 μg/ml) and _srb5_Δ (S, 450 μg/ml) holoenzymes and an activator (GST-VP16). Due to the low specific concentration of the _srb5_Δ holoenzyme in the MonoQ fraction (one-fifth the wild-type level), a threefold-larger amount of _srb5_Δ MonoQ fraction was injected to supply an amount of the _srb5_Δ holoenzyme comparable to that of the wild-type enzyme.
FIG. 4
Interactions of transcriptional activators with wild-type and mutant holoenzymes. (A, B, and D) Plotted is the change in the refractory index units (RU) versus time after injection of the indicated holoenzymes onto an activator-immobilized Biosensor chip. (A) SPR analysis of the interactions between Gal4VP16 and wild-type and _rgr1_Δ2 holoenzymes (70 μl of a MonoQ fraction at a concentration of 200 μg/ml). (B) SPR analysis of the interactions between wild-type (W) and _hrs1_Δ (H) holoenzymes (50 μl of a MonoQ fraction at a concentration of 250 μg/ml) and GST-VP16 or GCN4. (C) Coimmunoprecipitation (I.P.) of Gal4VP16 with wild-type (W) and mutant (R, _rgr1_Δ2) holoenzymes followed by Western blotting. Each holoenzyme (MonoQ fraction, 10 μg) was mixed with Gal4VP16 (300 ng) and immunoprecipitated with anti-Rgr1 antibody–beads (see Materials and Methods). In order to measure the amounts of precipitated Gal4VP16 and holoenzymes, we included equimolar amounts of recombinant Gal4VP16 and Med6 proteins (r-P; Med6, 80 ng; Gal4VP16, 60 ng) as quantitative standards. Immunoblot analyses with antisera specific for the proteins indicated to the right of the panel are shown. (D) SPR analysis of the interactions between wild-type (W, 150 μg/ml) and _srb5_Δ (S, 450 μg/ml) holoenzymes and an activator (GST-VP16). Due to the low specific concentration of the _srb5_Δ holoenzyme in the MonoQ fraction (one-fifth the wild-type level), a threefold-larger amount of _srb5_Δ MonoQ fraction was injected to supply an amount of the _srb5_Δ holoenzyme comparable to that of the wild-type enzyme.
FIG. 5
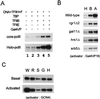
Direct interaction of Gal11 with acidic activators. (A) Far-Western analysis of holoenzymes with Gal4VP16. Wild-type (W) and _gal11_Δ (G) holoenzymes (immunopurified from a MonoQ fraction with an anti-Rgr1 antibody column, ∼2 μg each) were resolved on an SDS–10% polyacrylamide gel and transferred to a nitrocellulose membrane. The proteins were renatured and allowed to bind to 32P-labeled Gal4VP16 as described in Materials and Methods. The position of the Gal11 protein was revealed by Western analysis of the same blot with the use of anti-Gal11 antiserum (αGal11). (B) Column binding assay for interactions between Gal11 and acidic activators. Agarose beads conjugated with purified GST or GST-Gal11 (5 μg each) were incubated overnight at 4°C with 10 μl of in vitro-translated, 35S-labeled Gal4VP16, Gal4VP16Δ456FP442, or GCN4. After extensive washing, the beads were boiled in SDS sample buffer, and the proteins were resolved by SDS–12.5% PAGE and subjected to autoradiography. Load represents 10% of the amount of in vitro-translated products loaded on the beads.
FIG. 6
Simultaneous interactions of an activator with a holoenzyme and TBP. The ΔRU during the time course of the SPR analysis with a TBP-immobilized Biosensor chip is shown. Gal4VP16 (30 μg/ml, 30 μl) and wild-type holoenzyme (Holo-polII; 300 μg/ml, 50 μl) were injected sequentially after washing of the chip with binding buffer. The ΔRU for activator binding to TBP and that for holoenzyme binding to activator bound to TBP are shown.
Similar articles
- Activator-independent functions of the yeast mediator sin4 complex in preinitiation complex formation and transcription reinitiation.
Reeves WM, Hahn S. Reeves WM, et al. Mol Cell Biol. 2003 Jan;23(1):349-58. doi: 10.1128/MCB.23.1.349-358.2003. Mol Cell Biol. 2003. PMID: 12482986 Free PMC article. - Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme.
Kuras L, Struhl K. Kuras L, et al. Nature. 1999 Jun 10;399(6736):609-13. doi: 10.1038/21239. Nature. 1999. PMID: 10376605 - Recruitment of the RNA polymerase II holoenzyme and its implications in gene regulation.
Barberis A, Gaudreau L. Barberis A, et al. Biol Chem. 1998 Dec;379(12):1397-405. doi: 10.1515/bchm.1998.379.12.1397. Biol Chem. 1998. PMID: 9894806 Review. - [Interaction between yeast transcription factor GAL11 and general transcription factors].
Sakurai H, Fukasawa T. Sakurai H, et al. Tanpakushitsu Kakusan Koso. 1996 Jun;41(8 Suppl):1178-86. Tanpakushitsu Kakusan Koso. 1996. PMID: 8741639 Review. Japanese. No abstract available.
Cited by
- Tail and Kinase Modules Differently Regulate Core Mediator Recruitment and Function In Vivo.
Jeronimo C, Langelier MF, Bataille AR, Pascal JM, Pugh BF, Robert F. Jeronimo C, et al. Mol Cell. 2016 Nov 3;64(3):455-466. doi: 10.1016/j.molcel.2016.09.002. Epub 2016 Oct 20. Mol Cell. 2016. PMID: 27773677 Free PMC article. - Structural and functional characterization of PC2 and RNA polymerase II-associated subpopulations of metazoan Mediator.
Malik S, Baek HJ, Wu W, Roeder RG. Malik S, et al. Mol Cell Biol. 2005 Mar;25(6):2117-29. doi: 10.1128/MCB.25.6.2117-2129.2005. Mol Cell Biol. 2005. PMID: 15743810 Free PMC article. - The TRAP100 component of the TRAP/Mediator complex is essential in broad transcriptional events and development.
Ito M, Okano HJ, Darnell RB, Roeder RG. Ito M, et al. EMBO J. 2002 Jul 1;21(13):3464-75. doi: 10.1093/emboj/cdf348. EMBO J. 2002. PMID: 12093747 Free PMC article. - Activation domain-mediator interactions promote transcription preinitiation complex assembly on promoter DNA.
Cantin GT, Stevens JL, Berk AJ. Cantin GT, et al. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12003-8. doi: 10.1073/pnas.2035253100. Epub 2003 Sep 23. Proc Natl Acad Sci U S A. 2003. PMID: 14506297 Free PMC article. - RNA polymerase II (Pol II)-TFIIF and Pol II-mediator complexes: the major stable Pol II complexes and their activity in transcription initiation and reinitiation.
Rani PG, Ranish JA, Hahn S. Rani PG, et al. Mol Cell Biol. 2004 Feb;24(4):1709-20. doi: 10.1128/MCB.24.4.1709-1720.2004. Mol Cell Biol. 2004. PMID: 14749386 Free PMC article.
References
- Apone L M, Virbasius C M, Reese J C, Green M R. Yeast TAF(II)90 is required for cell-cycle progression through G2/M but not for general transcription activation. Genes Dev. 1996;10:2368–2380. - PubMed
- Berger S L, Cress W D, Cress A, Triezenberg S J, Guarente L. Selective inhibition of activated but not basal transcription by the acidic activation domain of VP16: evidence for transcriptional adaptors. Cell. 1990;61:1199–1208. - PubMed
- Bjorklund S, Kim Y J. Mediator of transcriptional regulation. Trends Biochem Sci. 1996;21:335–337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases