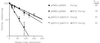Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein - PubMed (original) (raw)
Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein
D J Katzmann et al. Mol Cell Biol. 1999 Apr.
Abstract
The ATP binding cassette (ABC) transporter protein Yor1p was identified on the basis of its ability to elevate oligomycin resistance when it was overproduced from a high-copy-number plasmid. Analysis of the predicted amino acid sequence of Yor1p indicated that this protein was a new member of a subfamily of ABC transporter proteins defined by the multidrug resistance protein (MRP). In this work, Yor1p is demonstrated to localize to the Saccharomyces cerevisiae plasma membrane by both indirect immunofluorescence and biochemical fractionation studies. Several mutations were generated in the amino-terminal nucleotide binding domain (NBD1) of Yor1p to test if the high degree of sequence conservation in this region of the protein was important for function. Deletion of a phenylalanine residue at Yor1p position 670 led to a mutant protein that appeared to be retained in the endoplasmic reticulum (ER) and that was unstable. As shown by others, deletion of the analogous residue from a second mammalian MRP family member, the cystic fibrosis transmembrane conductance regulator (CFTR), also led to retention of this normally plasma membrane-localized protein in the ER. Changes in the spacing between or the sequences flanking functional motifs of Yor1p NBD1 led to defective trafficking or decreased activity of the mutant proteins. Analyses of the degradation of wild-type and DeltaF670 Yor1p indicated that the half-life of DeltaF670 Yor1p was dramatically shortened. While the vacuole was the primary site for turnover of wild-type Yor1p, degradation of DeltaF670 Yor1p was found to be more complex with both proteasomal and vacuolar contributions.
Figures
FIG. 1
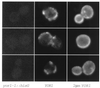
Yor1p localization by indirect immunofluorescence. Isogenic strains with various levels of the YOR1 gene were prepared for indirect immunofluorescence essentially as described previously (67). Strains carried the yor1-1::hisG allele (left panels), had a single copy of YOR1 (middle panels), or were transformed with the wild-type YOR1 gene carried on a 2 μm plasmid (right panels). Cells were labeled with affinity-purified rabbit anti-Yor1p C-terminal antibody, followed by incubation with fluorescein isothiocyanate-conjugated goat anti-rabbit antibody.
FIG. 2
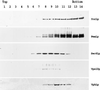
Biochemical fractionation of Yor1p. Wild-type cells were lysed with glass beads and fractionated over a sucrose gradient. Aliquots of each sucrose gradient fraction were precipitated with trichloroacetic acid, resuspended in Laemmli buffer, and electrophoresed by SDS-PAGE. The proteins were then transferred to nitrocellulose, and the resulting blot was probed with the indicated antisera. The relative positions of the light (top) and heavy (bottom) fractions of the sucrose gradient are indicated on the figure. Antisera employed listed on the right-hand side are as follows: Yor1p is the affinity-purified rabbit anti-Yor1p antibody used in Fig. 1, Pma1p corresponds to the plasma membrane ATPase protein (55), Sec61p is an integral membrane subunit of the translocon in the ER (59), Vps10p (also called Pep1p) is the Golgi apparatus- or prevacuole-localized carboxypeptidase Y (CPY) receptor (43), and Vph1p is the 100-kDa subunit of the vacuolar ATPase (42).
FIG. 3
Alignment of NBD1 regions from MRP family members. An alignment of the NBD1 segments from each of the indicated ABC transporter proteins is shown. The numbers refer to the respective positions along the polypeptide chain, and residues that are identical in at least three of the five selected proteins are boxed. The conserved structural motifs present in all ABC transporters are indicated by the heavy lines, and the sites of mutations in Yor1p NBD1 are denoted by arrows.
FIG. 4
Oligomycin resistance phenotypes of Yor1p and mutant variants. DKY7 (yor1-1::hisG) cells were transformed with low-copy-number plasmids bearing the genes that express the indicated forms of Yor1p or with the low-copy-number vector (pRS316). Transformants were grown to an _A_600 of approximately 1, and 1,000 cells were placed on YPGE medium containing a gradient of oligomycin (indicated by the wedge of increasing height). The plate was incubated at 30°C and photographed.
FIG. 5
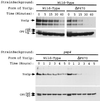
Steady-state levels of mutant Yor1p derivatives. Whole-cell extracts were prepared from DKY7 (yor1-1::hisG) cells expressing the indicated forms of Yor1p from low-copy-number plasmids. Protein (75 μg) was electrophoresed by SDS-PAGE, transferred to nitrocellulose, and blotted with anti-Yor1p (A) or anti-Vph1p (B) as a loading control.
FIG. 6
Subcellular fractionation of Yor1p NBD1 mutants. Cells lacking a chromosomal YOR1 locus (DKY7) were transformed with low-copy-number plasmids bearing the genes that express the indicated forms of Yor1p. Lysates were prepared and centrifuged through sucrose gradients as described in the legend to Fig. 2. Aliquots of each sucrose gradient were then subjected to Western blotting with the affinity-purified anti-Yor1p antibody (Yor1p) or the rabbit anti-Pma1p (Pma1p), rabbit anti-Vps10p (Vps10p), or rabbit anti-Sec61p (Sec61p) antiserum as listed on the right-hand side of each panel. The orientation of the sucrose gradient fractions is indicated as in Fig. 2.
FIG. 7
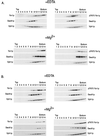
Vacuolar proteases influence the turnover of both wild-type and ΔF670 Yor1p. Isogenic yor1-1::hisG strains that either contained (top panels) or lacked (bottom panels) the PEP4 locus were transformed with low-copy-number plasmids bearing the gene that expresses the wild-type or ΔF670 form of Yor1p. Selected transformants were then analyzed by pulse-chase analysis followed by immunoprecipitation with the anti-Yor1p antiserum or an anti-CPY polyclonal antibody (from R. Piper). Levels of immunoprecipitated proteins were quantitated by phosphorimaging. The time scale for the pulse-chase in the PEP4 background was in minutes, while that for the pulse-chase with the pep4 strain was in hours. The position of Yor1p and the positions of the various forms of CPY (58) are denoted by arrows.
FIG. 8
Mg2+-dependent fractionation of ΔF670 Yor1p in PEP4 or pep4 cells. Lysates were prepared from PEP4 (A) or pep4 (B) cells expressing either wild-type or ΔF670 Yor1p. These lysates were subjected to sucrose gradient centrifugation in the presence or absence of Mg2+ as described previously (53). Aliquots of the gradient were then analyzed by Western blotting (see Materials and Methods) for the presence of either form of Yor1p and for Sec61p or Vph1p.
FIG. 9
Effect of loss of Sec12p function on turnover of wild-type and ΔF670 Yor1p. A strain containing the temperature-sensitive sec12-3 allele and lacking the YOR1 gene was constructed by one-step gene disruption of the YOR1 locus to produce EAE18. Low-copy-number plasmids bearing the gene that expresses either the wild-type or ΔF670 form of Yor1p were then introduced into this gene background. Turnover of these two forms of Yor1p was then assessed by pulse-chase immunoprecipitation analysis at the permissive (23°C) and restrictive (37°C) temperatures. The numbers refer to times in minutes after chase addition.
FIG. 10
ΔF670 but not wild-type Yor1p is responsive to changes in ubiquitin metabolism. (A) A strain lacking the PEP4 gene was transformed with a 2 μm plasmid containing a CUP1-UBI1 gene fusion along with low-copy-number plasmids bearing the gene for either wild-type or ΔF670 Yor1p. Levels of immunoprecipitable Yor1p were determined by pulse-chase analysis either in the absence (open symbols) or the presence (filled symbols) of copper sulfate to induce ubiquitin expression. The percentage of Yor1p remaining after chase was plotted as a function of time. The half-life (T1/2) of each immunoprecipitable Yor1p form is shown on the right-hand side in minutes. (B) An isogenic pair of yor1-1::hisG cells either lacking (ubc7) or containing (UBC7) an intact copy of chromosomal UBC7 was transformed with low-copy-number plasmids bearing the gene for wild-type or ΔF670 Yor1p. Yor1p stability was analyzed by pulse-chase analysis as described above. The filled symbols indicate the presence of UBC7, while the open symbols correspond to a strain carrying a ubc7-Δ1::HIS3 allele (32). The half-lives (in minutes) of the Yor1p forms are shown in the column on the right.
FIG. 11
A functional proteasome is required for rapid degradation of ΔF670 Yor1p. A yor1-1::hisG mutant cell lacking normal proteasome function (pre1-1 pre2-2) was transformed with low-copy-number plasmids bearing the gene for either the wild-type or ΔF670 form of Yor1p. Low-copy-number plasmids carrying PRE1 and PRE2 (pPRE1/pPRE2) or the vector plasmids alone (pRS313/pRS315) were introduced into these transformants to examine the consequence of varying the activity of the proteasome on turnover of these two forms of Yor1p. Appropriate transformants were analyzed for stability of wild-type or ΔF670 Yor1p by pulse-chase analysis as described above. The calculated half-lives (T1/2; in minutes) are on the right.
Similar articles
- Substitution of Yor1p NBD1 residues improves the thermal stability of Human Cystic Fibrosis Transmembrane Conductance Regulator.
Xavier BM, Hildebrandt E, Jiang F, Ding H, Kappes JC, Urbatsch IL. Xavier BM, et al. Protein Eng Des Sel. 2017 Oct 1;30(10):729-741. doi: 10.1093/protein/gzx054. Protein Eng Des Sel. 2017. PMID: 29053845 Free PMC article. - Intragenic suppressing mutations correct the folding and intracellular traffic of misfolded mutants of Yor1p, a eukaryotic drug transporter.
Pagant S, Halliday JJ, Kougentakis C, Miller EA. Pagant S, et al. J Biol Chem. 2010 Nov 19;285(47):36304-14. doi: 10.1074/jbc.M110.142760. Epub 2010 Sep 13. J Biol Chem. 2010. PMID: 20837481 Free PMC article. - Identification of interdependent signals required for anterograde traffic of the ATP-binding cassette transporter protein Yor1p.
Epping EA, Moye-Rowley WS. Epping EA, et al. J Biol Chem. 2002 Sep 20;277(38):34860-9. doi: 10.1074/jbc.M202987200. Epub 2002 Jul 9. J Biol Chem. 2002. PMID: 12107161 - ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily.
Paumi CM, Chuk M, Snider J, Stagljar I, Michaelis S. Paumi CM, et al. Microbiol Mol Biol Rev. 2009 Dec;73(4):577-93. doi: 10.1128/MMBR.00020-09. Microbiol Mol Biol Rev. 2009. PMID: 19946134 Free PMC article. Review. - Protein and peptide secretion by ABC exporters.
Wandersman C. Wandersman C. Res Microbiol. 1998 Mar;149(3):163-70. doi: 10.1016/s0923-2508(98)80076-4. Res Microbiol. 1998. PMID: 9766218 Review. No abstract available.
Cited by
- Dual action antifungal small molecule modulates multidrug efflux and TOR signaling.
Shekhar-Guturja T, Gunaherath GM, Wijeratne EM, Lambert JP, Averette AF, Lee SC, Kim T, Bahn YS, Tripodi F, Ammar R, Döhl K, Niewola-Staszkowska K, Schmitt L, Loewith RJ, Roth FP, Sanglard D, Andes D, Nislow C, Coccetti P, Gingras AC, Heitman J, Gunatilaka AA, Cowen LE. Shekhar-Guturja T, et al. Nat Chem Biol. 2016 Oct;12(10):867-75. doi: 10.1038/nchembio.2165. Epub 2016 Aug 29. Nat Chem Biol. 2016. PMID: 27571477 Free PMC article. - Genomic Data Mining Reveals Abundant Uncharacterized Transporters in Coccidioides immitis and Coccidioides posadasii.
Cai H, Zhang H, Guo DH, Wang Y, Gu J. Cai H, et al. J Fungi (Basel). 2022 Oct 10;8(10):1064. doi: 10.3390/jof8101064. J Fungi (Basel). 2022. PMID: 36294626 Free PMC article. - YBP1 and its homologue YBP2/YBH1 influence oxidative-stress tolerance by nonidentical mechanisms in Saccharomyces cerevisiae.
Gulshan K, Rovinsky SA, Moye-Rowley WS. Gulshan K, et al. Eukaryot Cell. 2004 Apr;3(2):318-30. doi: 10.1128/EC.3.2.318-330.2004. Eukaryot Cell. 2004. PMID: 15075262 Free PMC article. - A homolog of voltage-gated Ca(2+) channels stimulated by depletion of secretory Ca(2+) in yeast.
Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW. Locke EG, et al. Mol Cell Biol. 2000 Sep;20(18):6686-94. doi: 10.1128/MCB.20.18.6686-6694.2000. Mol Cell Biol. 2000. PMID: 10958666 Free PMC article. - Bro1 stimulates Vps4 to promote intralumenal vesicle formation during multivesicular body biogenesis.
Tseng CC, Dean S, Davies BA, Azmi IF, Pashkova N, Payne JA, Staffenhagen J, West M, Piper RC, Odorizzi G, Katzmann DJ. Tseng CC, et al. J Cell Biol. 2021 Aug 2;220(8):e202102070. doi: 10.1083/jcb.202102070. Epub 2021 Jun 23. J Cell Biol. 2021. PMID: 34160559 Free PMC article.
References
- Bakos E, Hegedus T, Hollo Z, Welker E, Tusnady G E, Zaman G J R, Flens M J, Varadi A, Sarkadi B. Membrane topology and glycosylation of the human multidrug resistance-associated protein. J Biol Chem. 1996;271:12322–12326. - PubMed
- Biederer T, Volkwein C, Sommer T. Role of Cue1p in ubiquitination and degradation at the ER surface. Science. 1997;278:1806–1809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials