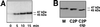cdk1- and cdk2-mediated phosphorylation of MyoD Ser200 in growing C2 myoblasts: role in modulating MyoD half-life and myogenic activity - PubMed (original) (raw)
cdk1- and cdk2-mediated phosphorylation of MyoD Ser200 in growing C2 myoblasts: role in modulating MyoD half-life and myogenic activity
M Kitzmann et al. Mol Cell Biol. 1999 Apr.
Abstract
We have examined the role of protein phosphorylation in the modulation of the key muscle-specific transcription factor MyoD. We show that MyoD is highly phosphorylated in growing myoblasts and undergoes substantial dephosphorylation during differentiation. MyoD can be efficiently phosphorylated in vitro by either purified cdk1-cyclin B or cdk1 and cdk2 immunoprecipitated from proliferative myoblasts. Comparative two-dimensional tryptic phosphopeptide mapping combined with site-directed mutagenesis revealed that cdk1 and cdk2 phosphorylate MyoD on serine 200 in proliferative myoblasts. In addition, when the seven proline-directed sites in MyoD were individually mutated, only substitution of serine 200 to a nonphosphorylatable alanine (MyoD-Ala200) abolished the slower-migrating hyperphosphorylated form of MyoD, seen either in vitro after phosphorylation by cdk1-cyclin B or in vivo following overexpression in 10T1/2 cells. The MyoD-Ala200 mutant displayed activity threefold higher than that of wild-type MyoD in transactivation of an E-box-dependent reporter gene and promoted markedly enhanced myogenic conversion and fusion of 10T1/2 fibroblasts into muscle cells. In addition, the half-life of MyoD-Ala200 protein was longer than that of wild-type MyoD, substantiating a role of Ser200 phosphorylation in regulating MyoD turnover in proliferative myoblasts. Taken together, our data show that direct phosphorylation of MyoD Ser200 by cdk1 and cdk2 plays an integral role in compromising MyoD activity during myoblast proliferation.
Figures
FIG. 1
Hyperphosphorylation of MyoD in C2.7 myoblasts. (A) Total cellular proteins were extracted from C2.7 cells and separated by 2D gel electrophoresis. The different isoforms of MyoD (arrows) were detected by Western blot analysis using extracts made from proliferative myoblasts (P) or cells placed for 60 h in differentiation medium or nuclear from extracts from proliferative myoblasts treated with CIP (P+cip). Migrations of the first dimension electrofocusing (IEF) gel (with the acid side indicated) and the second SDS-PAGE dimension are illustrated by arrows. (B) MyoD was immunoprecipitated from [32P]orthophosphate-labeled proliferative (Prol) or differentiated (Diff) C2.7 cells as described in Materials and Methods. MyoD was resolved by SDS-PAGE, its phosphorylation analyzed by autoradiography (32p), and the amount of immunoprecipitated MyoD protein was controlled by Western blot (WB) analysis.
FIG. 2
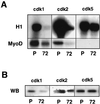
cdk1 and cdk2 from C2 myoblasts phosphorylate efficiently MyoD. (A) cdk1, cdk2, and cdk5 were immunoprecipitated from proliferative (P) and differentiated (72) C2.7 cells and assayed for kinase activity against H1 histone (top panel) and MyoD (bottom panel). Shown are autoradiograms of the different kinase reactions following SDS-PAGE. (B) Western blot (WB) analysis of cdk1, cdk2, and cdk5 after immunoprecipitation from proliferative (P) and differentiated (72) C2.7 cells.
FIG. 3
Electrophoretic shift of MyoD after phosphorylation by cdk1-cyclin B. (A) Bacterially produced MyoD protein was phosphorylated by purified cdk1-cyclin B kinase for the time indicated. Shown is the autoradiogram from the 32P phosphorylation reaction. Unphosphorylated MyoD migrates as 45-kDa band that shifts to 47 kDa in the course of phosphorylation by cdk1-cyclin B. (B) Western blot showing MyoD migration after SDS-PAGE of mitotic cells extracts (M) and proliferative C2.7 nuclear extracts before (C2P) and after (C2P cip) treatment with CIP.
FIG. 4
Phosphotryptic map analysis of MyoD phosphorylation in C2 myoblasts and following in vitro phosphorylation by cdk1-cyclin B. (A) Phosphorylation sites on MyoD were analyzed by tryptic digestion followed by 2D peptide mapping. The phosphopeptide map for in vivo MyoD phosphorylation (left panel) was obtained after immunoprecipitation of 32P-radiolabeled MyoD from dividing myoblasts. The phosphopeptide map for in vitro-phosphorylated MyoD (middle panel) was performed on bacterially produced MyoD phosphorylated in vitro by cdk1-cyclin B. In vivo and in vitro-phosphorylated MyoD were mixed following tryptic digestion, before 2D analysis (right panel). Numbers 1 and 2 indicate the two major phosphopeptides obtained from MyoD in proliferative myoblasts. The two major phosphopeptides found with MyoD phosphorylated in vitro are pointed to by arrows. (B) Mobility prediction of MyoD tryptic phosphopeptides by using the PhosPepSort program shows the pattern of migration for the four MyoD phosphopeptides that would be generated if all of the seven proline-directed sites present on MyoD were phosphorylated.
FIG. 5
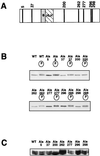
Among the seven potential CDK-dependent phosphorylation sites, mutation of only Ser200 prevents the phosphorylation shift of MyoD. (A) MyoD protein exhibits seven proline-directed sites on its amino acid sequence: Ser5, Ser37, Ser200, Ser262, Ser277, Thr296, and Ser298. Each of the Ser and Thr residues was mutated to Ala as described in Materials and Methods. (B) In vitro-translated 35S-labeled MyoD-wt (WT) and individual Ala mutants of MyoD (Ala5, Ala37, Ala200, Ala262, Ala277, Ala296, and Ala298) were incubated with (circled “p”) or without cdk1-cyclin B. Phosphorylation shifts were visualized after SDS-PAGE. Shown is the autoradiogram of the SDS-PAGE analysis. (C) Western blot analysis of MyoD overexpression in 10T1/2 cells transiently transfected with either MyoD-wt or each of the seven Ala mutants. Shown is the ECL detection of MyoD immunoreactivity.
FIG. 6
Phosphotryptic map analysis of MyoD-Ala5 and MyoD-Ala200 following in vitro phosphorylation by cdk1-cyclin B. Phosphorylation sites on MyoD were analyzed by tryptic digestion followed by 2D peptide mapping. Phosphopeptide maps were obtained for bacterially produced MyoD-wt (left panel), MyoD-Ala200 (middle panel), and MyoD-Ala5 (right panel) phosphorylated in vitro by cdk1-cyclin B. The two major phosphopeptides found with MyoD-wt phosphorylated in vitro are pointed to by arrows labeled Ser5 and Ser200.
FIG. 7
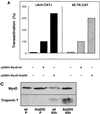
MyoD-Ala200 displays enhanced transactivating and myogenic activities. (A) pαAch-CAT+ and p4E-TK-CAT reporter constructs were cotransfected in 10T1/2 cells with either pEMSV or encoding plasmid pEMSV-MyoD-wt or pEMSV-MyoD-Ala200 and pCMV-βgal. Transfected cells were grown for 36 h in DMEM containing 10% FCS, and CAT activity was measured and corrected with respect to β-galactosidase activity. CAT activities are expressed relative to that of each reporter plasmid transfected with pEMSV-MyoD, set as 100%. (B) 10T1/2 were transfected with either pEMSV-MyoD-wt or pEMSV-MyoD-Ala200 and placed in differentiation medium (DMEM containing 2% FCS) for 60 h. Cells were fixed and stained for both MyoD and troponin T. Shown are the extents of myogenic conversion of 10T1/2 by MyoD-wt (left panels) and MyoD Ala200 (right panels) with staining for MyoD expression (a and b), troponin T expression (c and d), and DNA staining (e and f). Bar, 10 μm. The average percentages of cells expressing MyoD-wt or MyoD-Ala200 that coexpressed troponin T were calculated from two different experiments and are indicated in the bottom. The total numbers of MyoD-positive cells counted were 235 for MyoD-wt and 280 for MyoD-Ala200. (C) 10T1/2 cells were transfected with either pEMSV-MyoD-wt or pEMSV-MyoD-Ala200 as described above. Transfected cells were grown in proliferative medium (DMEM containing 10% FCS) for 24 h (P) and placed in differentiation medium for 60 h (60h). Cells were collected either before (P) or after (60h) myogenic conversion and analyzed by western blotting for MyoD and troponin T expression.
FIG. 7
MyoD-Ala200 displays enhanced transactivating and myogenic activities. (A) pαAch-CAT+ and p4E-TK-CAT reporter constructs were cotransfected in 10T1/2 cells with either pEMSV or encoding plasmid pEMSV-MyoD-wt or pEMSV-MyoD-Ala200 and pCMV-βgal. Transfected cells were grown for 36 h in DMEM containing 10% FCS, and CAT activity was measured and corrected with respect to β-galactosidase activity. CAT activities are expressed relative to that of each reporter plasmid transfected with pEMSV-MyoD, set as 100%. (B) 10T1/2 were transfected with either pEMSV-MyoD-wt or pEMSV-MyoD-Ala200 and placed in differentiation medium (DMEM containing 2% FCS) for 60 h. Cells were fixed and stained for both MyoD and troponin T. Shown are the extents of myogenic conversion of 10T1/2 by MyoD-wt (left panels) and MyoD Ala200 (right panels) with staining for MyoD expression (a and b), troponin T expression (c and d), and DNA staining (e and f). Bar, 10 μm. The average percentages of cells expressing MyoD-wt or MyoD-Ala200 that coexpressed troponin T were calculated from two different experiments and are indicated in the bottom. The total numbers of MyoD-positive cells counted were 235 for MyoD-wt and 280 for MyoD-Ala200. (C) 10T1/2 cells were transfected with either pEMSV-MyoD-wt or pEMSV-MyoD-Ala200 as described above. Transfected cells were grown in proliferative medium (DMEM containing 10% FCS) for 24 h (P) and placed in differentiation medium for 60 h (60h). Cells were collected either before (P) or after (60h) myogenic conversion and analyzed by western blotting for MyoD and troponin T expression.
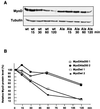
FIG. 8
Impeding MyoD Ser200 phosphorylation stabilizes the protein. (A) 10T1/2 cells were transfected with either pEMSV-MyoD-wt or pEMSV-MyoD-Ala200 and grown for 24 h in proliferative medium before addition of cycloheximide (15 μg/ml) to the medium for 0, 15, 30, 60, or 120 min. MyoD and α-tubulin protein levels were determined by immunoblot analysis at the indicated times after cycloheximide addition. (B) Immunoblots were quantified by densitometric scanning, and MyoD protein levels (corrected with respect to tubulin expression) were expressed relative to that observed before cycloheximide treatment, set as 100%.
Similar articles
- p57(Kip2) stabilizes the MyoD protein by inhibiting cyclin E-Cdk2 kinase activity in growing myoblasts.
Reynaud EG, Pelpel K, Guillier M, Leibovitch MP, Leibovitch SA. Reynaud EG, et al. Mol Cell Biol. 1999 Nov;19(11):7621-9. doi: 10.1128/MCB.19.11.7621. Mol Cell Biol. 1999. PMID: 10523650 Free PMC article. - Cyclin E-cdk2 phosphorylation promotes late G1-phase degradation of MyoD in muscle cells.
Tintignac LA, Leibovitch MP, Kitzmann M, Fernandez A, Ducommun B, Meijer L, Leibovitch SA. Tintignac LA, et al. Exp Cell Res. 2000 Aug 25;259(1):300-7. doi: 10.1006/excr.2000.4973. Exp Cell Res. 2000. PMID: 10942602 - Stabilization of MyoD by direct binding to p57(Kip2).
Reynaud EG, Leibovitch MP, Tintignac LA, Pelpel K, Guillier M, Leibovitch SA. Reynaud EG, et al. J Biol Chem. 2000 Jun 23;275(25):18767-76. doi: 10.1074/jbc.M907412199. J Biol Chem. 2000. PMID: 10764802 - Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts.
Kitzmann M, Fernandez A. Kitzmann M, et al. Cell Mol Life Sci. 2001 Apr;58(4):571-9. doi: 10.1007/PL00000882. Cell Mol Life Sci. 2001. PMID: 11361092 Free PMC article. Review. - [The characterization of human cdc2 kinase and CDK2].
Yasuda H, Kamijo M, Ohba Y. Yasuda H, et al. Yakugaku Zasshi. 1993 Dec;113(12):829-46. doi: 10.1248/yakushi1947.113.12_829. Yakugaku Zasshi. 1993. PMID: 8301538 Review. Japanese.
Cited by
- Mutant DMPK 3'-UTR transcripts disrupt C2C12 myogenic differentiation by compromising MyoD.
Amack JD, Reagan SR, Mahadevan MS. Amack JD, et al. J Cell Biol. 2002 Nov 11;159(3):419-29. doi: 10.1083/jcb.200206020. Epub 2002 Nov 11. J Cell Biol. 2002. PMID: 12427866 Free PMC article. - Proline isomerase Pin1 represses terminal differentiation and myocyte enhancer factor 2C function in skeletal muscle cells.
Magli A, Angelelli C, Ganassi M, Baruffaldi F, Matafora V, Battini R, Bachi A, Messina G, Rustighi A, Del Sal G, Ferrari S, Molinari S. Magli A, et al. J Biol Chem. 2010 Nov 5;285(45):34518-27. doi: 10.1074/jbc.M110.104133. Epub 2010 Aug 27. J Biol Chem. 2010. PMID: 20801874 Free PMC article. - The Presence of Two MyoD Genes in a Subset of Acanthopterygii Fish Is Associated with a Polyserine Insert in MyoD1.
White LJ, Russell AJ, Pizzey AR, Dasmahapatra KK, Pownall ME. White LJ, et al. J Dev Biol. 2023 Apr 28;11(2):19. doi: 10.3390/jdb11020019. J Dev Biol. 2023. PMID: 37218813 Free PMC article. - Biochemical characterization of the feedforward loop between CDK1 and FOXM1 in epidermal stem cells.
Polito MP, Romaldini A, Tagliazucchi L, Marini G, Radice F, Gozza GA, Bergamini G, Costi MP, Enzo E. Polito MP, et al. Biol Direct. 2024 Oct 13;19(1):91. doi: 10.1186/s13062-024-00540-8. Biol Direct. 2024. PMID: 39396994 Free PMC article. - p57(Kip2) stabilizes the MyoD protein by inhibiting cyclin E-Cdk2 kinase activity in growing myoblasts.
Reynaud EG, Pelpel K, Guillier M, Leibovitch MP, Leibovitch SA. Reynaud EG, et al. Mol Cell Biol. 1999 Nov;19(11):7621-9. doi: 10.1128/MCB.19.11.7621. Mol Cell Biol. 1999. PMID: 10523650 Free PMC article.
References
- Akhurst R J, Flavin N B, Worden J, Lee M G. Intracellular localisation and expression of mammalian CDC2 protein during myogenic differentiation. Differentiation. 1989;40:36–41. - PubMed
- Benezra R, Davis R L, Lockshon D, Turner D L, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous