Interleukin-10 and antigen-presenting cells actively suppress Th1 cells in BALB/c mice infected with the filarial parasite Brugia pahangi - PubMed (original) (raw)
Interleukin-10 and antigen-presenting cells actively suppress Th1 cells in BALB/c mice infected with the filarial parasite Brugia pahangi
J Osborne et al. Infect Immun. 1999 Apr.
Free PMC article
Abstract
Infection with the third-stage larvae (L3) of the filarial nematode Brugia results in a Th2-biased immune response in mice and humans. Previously we have shown that the production of interleukin 4 (IL-4) is critical for down-regulating polyclonal Th1 responses in L3-infected mice. However, the in vitro neutralization of IL-4 did not fully recover the defective polyclonal Th1 responses, nor did it result in the production of any antigen (Ag)-specific Th1 cytokines, suggesting that perhaps infection with L3 does not result in priming of Th1 cells in vivo. In this study, we analyzed the role of IL-10 and Ag-presenting cells (APCs) in the spleen as additional factors controlling the Th2 bias in infected mice. Our data show that IL-10 and APCs also contribute to the suppression of mitogen-driven Th1 responses of spleen cells from infected mice. In addition, the neutralization of IL-10 or the replacement of the resident APC population from spleen cell cultures resulted in the production of Ag-specific Th1 cytokines. Irradiated spleen cells from either L3-infected or uninfected mice were able to restore Ag-specific Th1 responses in vitro. Therefore, it appears that Brugia-reactive Th1 cells are primed following infection with L3, but are actively suppressed in vivo by a mechanism that involves IL-10 and the resident APC population, but not IL-4. These results indicate that a complex interplay of cytokines and cell populations underscores the Th2-polarized response in L3-infected mice.
Figures
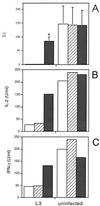
FIG. 1
Effect of neutralizing anti-IL-10 MAb on ConA-driven responses of spleen cells from mice infected with L3. Mice were injected s.c. with 50 L3 or HBSS, and spleens were removed at day 12 p.i. ConA-stimulated cultures were incubated alone (□) or with 10 μg of either an isotype-matched control (R59-40) (▨) or anti-IL-10 (JES5-2A5) ( ) per ml. After 48 h of incubation, proliferation (A) and IL-2 (B) and IFN-γ (C) production were measured. (A) The means of triplicate wells are expressed as SIs (cpm with ConA/cpm with medium alone). The SIs represent the means ± standard deviations of five animals per group, ∗, significant difference (P < 0.05) between the values obtained with JES5-2A5 and R59-40. SIs were also significantly different (P < 0.05) between the cells of L3-infected mice treated with JES5-2A5 and uninfected mice with no treatment. (B) and (C) Cytokine assays were performed with spleen cells pooled from five animals in each group. The results presented were comparable in two additional experiments.
) per ml. After 48 h of incubation, proliferation (A) and IL-2 (B) and IFN-γ (C) production were measured. (A) The means of triplicate wells are expressed as SIs (cpm with ConA/cpm with medium alone). The SIs represent the means ± standard deviations of five animals per group, ∗, significant difference (P < 0.05) between the values obtained with JES5-2A5 and R59-40. SIs were also significantly different (P < 0.05) between the cells of L3-infected mice treated with JES5-2A5 and uninfected mice with no treatment. (B) and (C) Cytokine assays were performed with spleen cells pooled from five animals in each group. The results presented were comparable in two additional experiments.
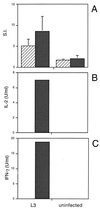
FIG. 2
Effect of neutralizing anti-IL-10 MAb on Ag-driven responses of spleen cells from mice infected with L3. Mice were injected as described in the legend to Fig. 1. Ag-stimulated cultures were incubated with 10 μg of either an isotype-matched control (R59-40) (▨) or anti-IL-10 (JES5-2A5) (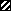 ) per ml, and proliferation (A) and IL-2 (B) and IFN-γ (C) production were measured, as described previously. (A) The SIs shown are the means ± standard deviations of five animals per group. (B and C) Cytokine assays were performed with spleen cells pooled from five animals in each group. The results presented were comparable in two additional experiments.
) per ml, and proliferation (A) and IL-2 (B) and IFN-γ (C) production were measured, as described previously. (A) The SIs shown are the means ± standard deviations of five animals per group. (B and C) Cytokine assays were performed with spleen cells pooled from five animals in each group. The results presented were comparable in two additional experiments.
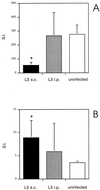
FIG. 3
Proliferative responses of spleen cells from BALB/c mice following s.c. or i.p. injection with L3 to ConA and B. pahangi adult Ag. Mice were injected s.c. or i.p. with 50 L3. Uninfected controls were injected s.c. with HBSS. At day 12 p.i., proliferation of spleen cells from the three groups of mice to ConA (A) or B. pahangi adult Ag (B) was measured. The SIs represent the means ± standard deviations of five animals per group. ∗, significant difference (P < 0.05) compared to uninfected controls.
FIG. 4
Effect of depletion of nylon wool-adherent cells from spleens of L3-infected mice on mitogen-driven responses. Mice were injected as described in the legend to Fig. 1. Spleen cells from five mice in each group were pooled. Splenic T cells from L3-infected mice, purified by passing the pooled spleen cell suspension over nylon wool columns, were incubated with irradiated syngeneic spleen cells from uninfected mice (▨) or with irradiated spleen cells from L3-infected mice (▩). ConA-stimulated proliferation (A) and cytokine production (B to F) were measured in these cultures and in cultures of unseparated spleen cells from L3-infected ( ) and uninfected (
) and uninfected ( ) mice.
) mice.
FIG. 5
Effect of depletion of nylon wool-adherent cells from spleens of L3-infected mice on Ag-driven responses. Mice were injected as described in the legend to Fig. 1. Splenic T cells from L3-infected mice, purified by passing the pooled spleen cell suspension over nylon wool columns, were incubated with irradiated syngeneic spleen cells from uninfected mice (▨) or with irradiated spleen cells from L3-infected mice (▩). Ag-stimulated proliferation (A) and cytokine production (B to F) were measured in these cultures and in cultures of unseparated spleen cells from L3-infected ( ) and uninfected (
) and uninfected ( ) mice.
) mice.
Similar articles
- Anti-interleukin-4 modulation of the Th2 polarized response to the parasitic nematode Brugia pahangi.
Osborne J, Hunter SJ, Devaney E. Osborne J, et al. Infect Immun. 1996 Sep;64(9):3461-6. doi: 10.1128/iai.64.9.3461-3466.1996. Infect Immun. 1996. PMID: 8751885 Free PMC article. - Regulatory T cells modulate Th2 responses induced by Brugia pahangi third-stage larvae.
Gillan V, Devaney E. Gillan V, et al. Infect Immun. 2005 Jul;73(7):4034-42. doi: 10.1128/IAI.73.7.4034-4042.2005. Infect Immun. 2005. PMID: 15972491 Free PMC article. - Infection outcome and cytokine gene expression in Brugia pahangi- infected gerbils (Meriones unguiculatus) sensitized with Brucella abortus.
Chirgwin SR, Elzer PH, Coleman SU, Nowling JM, Hagius SD, Edmonds MD, Klei TR. Chirgwin SR, et al. Infect Immun. 2002 Nov;70(11):5938-45. doi: 10.1128/IAI.70.11.5938-5945.2002. Infect Immun. 2002. PMID: 12379668 Free PMC article. - The role of CD4 cells in protective immunity to Brugia pahangi.
Bancroft AJ, Grencis RK, Else KJ, Devaney E. Bancroft AJ, et al. Parasite Immunol. 1994 Jul;16(7):385-7. doi: 10.1111/j.1365-3024.1994.tb00364.x. Parasite Immunol. 1994. PMID: 7970877 - Ocular Brugia pahangi Filariasis Complicated by Severe Macular Damage in Thailand: Case Report and Literature Review.
Suphap N, Somkijrungroj T, Kongwattananon W, Supawatjariyakul W, Pataradool T, Kraivichian K, Jantarabenjakul W, Tulvatana W, Preativatanyou K. Suphap N, et al. Am J Trop Med Hyg. 2024 Apr 30;110(6):1158-1164. doi: 10.4269/ajtmh.24-0047. Print 2024 Jun 5. Am J Trop Med Hyg. 2024. PMID: 38688273 Review.
Cited by
- Both free-living and parasitic nematodes induce a characteristic Th2 response that is dependent on the presence of intact glycans.
Tawill S, Le Goff L, Ali F, Blaxter M, Allen JE. Tawill S, et al. Infect Immun. 2004 Jan;72(1):398-407. doi: 10.1128/IAI.72.1.398-407.2004. Infect Immun. 2004. PMID: 14688121 Free PMC article. - Molecular Characterization of a Dirofilaria immitis Cysteine Protease Inhibitor (Cystatin) and Its Possible Role in Filarial Immune Evasion.
Dong X, Xu J, Song H, Liu Y, Wu M, Zhang H, Jing B, Lai W, Gu X, Xie Y, Peng X, Yang G. Dong X, et al. Genes (Basel). 2019 Apr 12;10(4):300. doi: 10.3390/genes10040300. Genes (Basel). 2019. PMID: 31013806 Free PMC article. - Infective Larvae of Brugia malayi Induce Polarization of Host Macrophages that Helps in Immune Evasion.
Sharma A, Sharma P, Ganga L, Satoeya N, Mishra S, Vishwakarma AL, Srivastava M. Sharma A, et al. Front Immunol. 2018 Feb 12;9:194. doi: 10.3389/fimmu.2018.00194. eCollection 2018. Front Immunol. 2018. PMID: 29483912 Free PMC article. - Parasite-specific immunomodulatory functions of filarial cystatin.
Schierack P, Lucius R, Sonnenburg B, Schilling K, Hartmann S. Schierack P, et al. Infect Immun. 2003 May;71(5):2422-9. doi: 10.1128/IAI.71.5.2422-2429.2003. Infect Immun. 2003. PMID: 12704112 Free PMC article. - Biochemical and immunological characterization of annexin B30 from Clonorchis sinensis excretory/secretory products.
He L, Ren M, Chen X, Wang X, Li S, Lin J, Liang C, Liang P, Hu Y, Lei H, Bian M, Huang Y, Wu Z, Li X, Yu X. He L, et al. Parasitol Res. 2014 Jul;113(7):2743-55. doi: 10.1007/s00436-014-3935-4. Epub 2014 May 27. Parasitol Res. 2014. PMID: 24861011
References
- Albina J E, Abate J A, Henry W L., Jr Nitric oxide production is required for murine resident macrophages to suppress mitogen-stimulated T cell proliferation. J Immunol. 1991;147:144–148. - PubMed
- Allen J E, Lawrence R A, Maizels R M. APC from mice harbouring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int Immunol. 1996;8:143–151. - PubMed
- Barral-Netto M, Barral A, Brownell C E, Skeiky Y A W, Ellingsworth L R, Twardzik D R, Reed S G. Transforming growth factor-B in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–548. - PubMed
- Bretscher P A, Wei G, Menon J N, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous