Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology - PubMed (original) (raw)
Comparative Study
Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology
C A Gutekunst et al. J Neurosci. 1999.
Abstract
The data we report in this study concern the types, location, numbers, forms, and composition of microscopic huntingtin aggregates in brain tissues from humans with different grades of Huntington's disease (HD). We have developed a fusion protein antibody against the first 256 amino acids that preferentially recognizes aggregated huntingtin and labels many more aggregates in neuronal nuclei, perikarya, and processes in human brain than have been described previously. Using this antibody and human brain tissue ranging from presymptomatic to grade 4, we have compared the numbers and locations of nuclear and neuropil aggregates with the known patterns of neuronal death in HD. We show that neuropil aggregates are much more common than nuclear aggregates and can be present in large numbers before the onset of clinical symptoms. There are also many more aggregates in cortex than in striatum, where they are actually uncommon. Although the striatum is the most affected region in HD, only 1-4% of striatal neurons in all grades of HD have nuclear aggregates. Neuropil aggregates, which we have identified by electron microscopy to occur in dendrites and dendritic spines, could play a role in the known dendritic pathology that occurs in HD. Aggregates increase in size in advanced grades, suggesting that they may persist in neurons that are more likely to survive. Ubiquitination is apparent in only a subset of aggregates, suggesting that ubiquitin-mediated proteolysis of aggregates may be late or variable.
Figures
Fig. 1.
Antibody EM48 and its reaction with N-terminal fragments of huntingtin. A, Schematic structure showing a truncated human huntingtin cDNA generated by PCR. Its corresponding region in wild-type huntingtin cDNA is shown above. The truncated protein contains only two glutamines in the glutamine repeat region and deletes the polyproline stretches. The numbers in parentheses represent the number of glutamines (Q) or prolines in normal human huntingtin.B, Western blots showing that EM48 recognizes native huntingtin (350 kDa band) in human brain cortex. C, EM48 immunofluorescent staining of 293 cells transfected with a series of cDNA constructs encoding different N-terminal fragments of human huntingtin with 120 glutamine repeats. The numbers in parentheses are N-terminal amino acid residues not including glutamine repeats. Note that only the huntingtin fragment (1–311) forms aggregates.
Fig. 2.
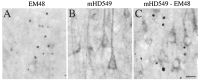
Neuropil aggregates are not labeled by an antibody to the internal region of huntingtin. Adjacent sections through cerebral cortex from a grade 1 HD case were immunostained with (A) EM48 alone, (B) mHD549 alone, or (C) EM48 and mHD549 combined. mHD549 staining is found in the perikarya and proximal dendrites of the pyramidal neurons (A, B) but is not found in aggregates. In contrast, EM48 intensely labels the aggregates (arrowheads). Scale bar, 30 μm.
Fig. 3.
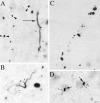
Types of EM48-immunoreactive aggregates. Light micrographs showing EM48-labeled aggregates of different shapes and cellular localization in HD cortex. Aggregates were found in the neuropil (A–C) and in neuronal nuclei (D, long arrows) and perikarya (D, small arrows). In the neuropil, small spherical or fusiform aggregates were either scattered (A, arrowheads) or arranged in linear arrays (C) reminiscent of neuronal process. EM48 immunoreactivity is also found in long tubular (A, long arrow) or serpentine elements (B) reminiscent of short dendritic segments and branch points, some of which appeared to give rise to immunoreactive dendritic spines (A, B, small arrows). Scale bar (shown in_D_ for A–D), 10 μm.
Fig. 4.
Neuropil aggregates in dendritic profiles. Electron micrographs of EM48 immunogold-labeled aggregates in insular cortex from an adult HD brain of grade 1. Immunogold particles are associated with aggregates made of filamentous material within dendritic processes. A is an example of a large caliber dendrite (d) containing an immunolabeled aggregate. Mitochondria (arrows) are seen in the cytoplasm adjacent to the aggregate. B shows a labeled aggregate in a dendritic spine receiving synaptic contact (arrow) from an axon terminal (a). In C and D, labeled aggregates are shown in longitudinal sections through two dendrites. In C, the filaments constituting the aggregates align with the orientation of the dendrite. Near the aggregates, the dendrites are receiving synaptic contact (arrow) from an axon terminal (a). Synaptic vesicles can be seen in the axon terminals (B, C). Scale bars, 500 nm.
Fig. 5.
Huntingtin aggregates in human postmortem cerebral cortex and striatum from a presymptomatic case. Light micrographs are from the insular cortex (A) and dorsal striatum (B). Large numbers of EM48-immunoreactive aggregates of a wide variety of shapes and sizes are visible in cortex. All of these aggregates are in the neuropil. In contrast, striatal aggregates are exceedingly uncommon. Scale bar, 70 μm.
Fig. 6.
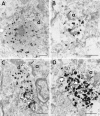
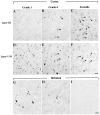
Neuropil and nuclear aggregates in cortex and striatum. Micrographs of coronal sections through the top layer (layer III, top panel) and the bottom layers (layers V/VI, middle panel) of insular cortex from adult HD brain of grade 1 (A, D, G), grade 4 (B, E, H), or juvenile HD brain (C, F). In the cortex of the grade 1 HD brain, neuropil aggregates are more frequent in layers V/VI than in layer III. More nuclear aggregates (arrowheads) are present in grade 4 and juvenile HD brain. Layer III from grade 4 adult HD brain has more aggregates than from grade 1. In the striatum, EM48-immunoreactive aggregates are present in grade 1 (G) and grade 4 (H), but at a much lower density than in cortex. Aggregates were absent in controls (I). Arrowheads indicate intranuclear aggregates. Scale bar, 50 μm.
Fig. 7.
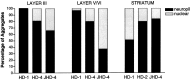
Quantitative analysis of neuropil aggregates in HD brains. This graph shows the percentages of neuropil and nuclear aggregates in layers III and V/VI of insular cortex and caudate nucleus from grade 1, grade 4, and juvenile HD cases. The proportion of aggregates that are in the neuropil decreases with disease grade in cortex but increases with disease grade in striatum.
Fig. 8.
The frequency of aggregates of various sizes in grade 1 and grade 4 cases. This graph shows the relative frequencies of aggregates based on their size using increments in their area of 15 nm2. Small aggregates are more frequent in earlier grade cases.
Fig. 9.
Ubiquitination of EM48 aggregates. Adjacent sections through layers V/VI of HD cerebral cortex immunolabeled with EM48 (A, B) and ubiquitin (C, D). In grade 1, the density of EM48-immunostained aggregates is three times that of ubiquitin-labeled aggregates (A, C). In grade 4, however, the densities are more similar (B, D). At higher magnification, it is evident that small perikaryal aggregates are stained by (E) EM48 but not by (F) anti-ubiquitin antibody. Scale bars:A–D, 70 μm; E, F, 10 μm.
Similar articles
- Huntington aggregates may not predict neuronal death in Huntington's disease.
Kuemmerle S, Gutekunst CA, Klein AM, Li XJ, Li SH, Beal MF, Hersch SM, Ferrante RJ. Kuemmerle S, et al. Ann Neurol. 1999 Dec;46(6):842-9. Ann Neurol. 1999. PMID: 10589536 - Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington's disease with 140 CAG repeats.
Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF. Menalled LB, et al. J Comp Neurol. 2003 Oct 6;465(1):11-26. doi: 10.1002/cne.10776. J Comp Neurol. 2003. PMID: 12926013 - Ultrastructural localization and progressive formation of neuropil aggregates in Huntington's disease transgenic mice.
Li H, Li SH, Cheng AL, Mangiarini L, Bates GP, Li XJ. Li H, et al. Hum Mol Genet. 1999 Jul;8(7):1227-36. doi: 10.1093/hmg/8.7.1227. Hum Mol Genet. 1999. PMID: 10369868 - The early cellular pathology of Huntington's disease.
Li XJ. Li XJ. Mol Neurobiol. 1999 Oct-Dec;20(2-3):111-24. doi: 10.1007/BF02742437. Mol Neurobiol. 1999. PMID: 10966117 Review. - The selective vulnerability of nerve cells in Huntington's disease.
Sieradzan KA, Mann DM. Sieradzan KA, et al. Neuropathol Appl Neurobiol. 2001 Feb;27(1):1-21. doi: 10.1046/j.0305-1846.2001.00299.x. Neuropathol Appl Neurobiol. 2001. PMID: 11298997 Review.
Cited by
- N17 Modifies mutant Huntingtin nuclear pathogenesis and severity of disease in HD BAC transgenic mice.
Gu X, Cantle JP, Greiner ER, Lee CY, Barth AM, Gao F, Park CS, Zhang Z, Sandoval-Miller S, Zhang RL, Diamond M, Mody I, Coppola G, Yang XW. Gu X, et al. Neuron. 2015 Feb 18;85(4):726-41. doi: 10.1016/j.neuron.2015.01.008. Epub 2015 Feb 5. Neuron. 2015. PMID: 25661181 Free PMC article. - Huntingtin contains an ubiquitin-binding domain and regulates lysosomal targeting of mitochondrial and RNA-binding proteins.
Fote GM, Eapen VV, Lim RG, Yu C, Salazar L, McClure NR, McKnight J, Nguyen TB, Heath MC, Lau AL, Villamil MA, Miramontes R, Kratter IH, Finkbeiner S, Reidling JC, Paulo JA, Kaiser P, Huang L, Housman DE, Thompson LM, Steffan JS. Fote GM, et al. Proc Natl Acad Sci U S A. 2024 Aug 6;121(32):e2319091121. doi: 10.1073/pnas.2319091121. Epub 2024 Jul 29. Proc Natl Acad Sci U S A. 2024. PMID: 39074279 Free PMC article. - Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington's disease.
Gu X, André VM, Cepeda C, Li SH, Li XJ, Levine MS, Yang XW. Gu X, et al. Mol Neurodegener. 2007 Apr 30;2:8. doi: 10.1186/1750-1326-2-8. Mol Neurodegener. 2007. PMID: 17470275 Free PMC article. - The Huntington's disease protein interacts with p53 and CREB-binding protein and represses transcription.
Steffan JS, Kazantsev A, Spasic-Boskovic O, Greenwald M, Zhu YZ, Gohler H, Wanker EE, Bates GP, Housman DE, Thompson LM. Steffan JS, et al. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6763-8. doi: 10.1073/pnas.100110097. Proc Natl Acad Sci U S A. 2000. PMID: 10823891 Free PMC article. - Monkey hybrid stem cells develop cellular features of Huntington's disease.
Laowtammathron C, Cheng ECh, Cheng PH, Snyder BR, Yang SH, Johnson Z, Lorthongpanich C, Kuo HC, Parnpai R, Chan AW. Laowtammathron C, et al. BMC Cell Biol. 2010 Feb 5;11:12. doi: 10.1186/1471-2121-11-12. BMC Cell Biol. 2010. PMID: 20132560 Free PMC article.
References
- Aylward EH, Anderson NB, Bylsma FW, Wagster MV, Barta PE, Sherr M, Feeney J, Davis A, Rosenblatt A, Pearlson GD, Ross CA. Frontal lobe volume in patients with Huntington’s disease. Neurology. 1998;50:252–258. - PubMed
- Becher MW, Kotzuk JA, Sharp AH, Davies SW, Bates GP, Price DL, Ross CA. Intranuclear neuronal inclusions in Huntington’s disease and dentatorubral and pallidoluysian atrophy: correlation between the density of inclusions and IT15 CAG triplet repeat length. Neurobiol Dis. 1998;4:387–397. - PubMed
- Cooper J, Schilling G, Peters M, Herring W, Sharp A, Kaminsky Z, Masone J, Khan F, Delanoy M, Borchelt D, Dawson V, Dawson T, Ross C. Truncated N-terminal fragments of huntingtin with expanded glutamine repeats form nuclear and cytoplasmic aggregates in cell culture. Hum Mol Genet. 1998;7:783–790. - PubMed
- Cudkowicz M, Kowall NW. Degeneration of pyramidal projection neurons in Huntington’s disease cortex. Ann Neurol. 1990;27:200–204. - PubMed
- Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY. Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat Genet. 1998;19:148–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous