Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats - PubMed (original) (raw)
Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats
R M Sullivan et al. J Neurosci. 1999.
Abstract
The medial prefrontal cortex (mPFC) is highly activated by stress and modulates neuroendocrine and autonomic function. Dopaminergic inputs to mPFC facilitate coping ability and demonstrate considerable hemispheric functional lateralization. The present study investigated the potentially lateralized regulation of stress responses at the level of mPFC output neurons, using ibotenic acid lesions. Neuroendocrine function was assessed by plasma corticosterone increases in response to acute or repeated 20 min restraint stress. The primary index of autonomic activation was gastric ulcer development during a separate cold restraint stress. Restraint-induced defecation was also monitored. Plasma corticosterone levels were markedly lower in response to repeated versus acute restraint stress. In acutely restrained animals, right or bilateral, but not left mPFC lesions, decreased prestress corticosterone levels, whereas in repeatedly restrained rats, the same lesions significantly reduced the peak stress-induced corticosterone response. Stress ulcer development (after a single cold restraint stress) was greatly reduced by either right or bilateral mPFC lesions but was unaffected by left lesions. Restraint-induced defecation was elevated in animals with left mPFC lesions. Finally, a left-biased asymmetry in adrenal gland weights was observed across animals, which was unaffected by mPFC lesions. The results suggest that mPFC output neurons demonstrate an intrinsic right brain specialization in both neuroendocrine and autonomic activation. Such findings may be particularly relevant to clinical depression which is associated with both disturbances in stress regulatory systems and hemispheric imbalances in prefrontal function.
Figures
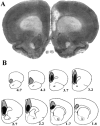
Fig. 1.
Extent of ibotenate-induced cell loss in mPFC-lesioned rats. A depicts the typical degree of cell loss in infralimbic, prelimbic, and cingulate cortex, in this case in a right-lesioned animal. In B,black and shaded regions respectively represent the minimum and maximum extent of cell loss across animals.Numbers represent distance (in millimeters) anterior to bregma. See Results for additional detail.
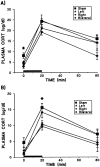
Fig. 2.
Effects of mPFC ibotenate lesions and treatments on mean (± SEM) plasma CORT levels in acutely (A) and repeatedly (B) restrained rats. The treatment difference in magnitude of CORT levels is reflected by the different scales of the two figures, because the CORT levels of repeated restraint groups were approximately half those of acute restraint animals at all time points, indicating a marked HPA adaptation to this stressor. Solid bars depict the 20 min period of restraint stress (room temperature). In acutely restrained animals, significant main effects were found for both lesion (F(3,27) = 5.18; p = 0.006) and sample (F(2,54) = 87.45;p < 0.0005), with no lesion × sample interaction. Although mPFC lesions tended to suppress CORT levels across samples, group differences were only significant at the prestress time point, because both right and bilateral groups were reduced relative to shams (Tukey’s post hoc analysis; *p < 0.05). As with acute restraint, repeated restraint treatment revealed significant effects for lesion (F(3,24) = 5.66; p = 0.004) and sample (F(2,48) = 58.45;p < 0.0005) with no interaction. In this case, significant group differences were seen only in peak CORT levels, because both right and bilateral groups were significantly suppressed relative to shams (Tukey’s post hoc analysis; *p < 0.05).
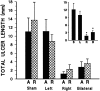
Fig. 3.
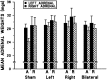
Mean total ulcer length (± SEM) in mPFC-lesioned rats induced by a single 2.5 hr cold restraint session. A significant effect of mPFC lesion was observed (F(3,58)= 8.98; p < 0.0005), with no significant effect of treatment and no lesion × treatment interaction. Regardless of previous experience with acute (A) or repeated (R) 20 min restraint stress, bilateral and right lesion groups each differed significantly from both the shams and left-lesioned animals (Tukey’s post hoc analysis; *p < 0.05 in each case). Left-lesioned rats did not differ from shams, nor did right-lesioned rats differ from the bilateral lesion group. The inset reveals these pronounced lesion effect with treatments collapsed. S,L, R, and B refer to sham, left, right, and bilateral mPFC lesion groups, respectively.
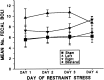
Fig. 4.
Mean defecation in response to daily 20 min restraint sessions. A significant lesion effect was found on this measure (F(3,24) = 6.82;p = 0.002), with no effect for days and no interaction for lesion × days. Rats with left mPFC lesions defecated to a greater extent than all other groups across days of testing.
Fig. 5.
Left/right asymmetry in mean adrenal weights. A significant main effect was observed for side (F(1,51) = 7.56; p = 0.008), because the left adrenal was larger across animals. There were no significant effects for either lesion or treatment on this gross measure of neuroendocrine status, and no significant interactions were found. The A and R represent acute and repeated restraint treatment conditions, respectively.
Similar articles
- The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor.
Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. Figueiredo HF, et al. Eur J Neurosci. 2003 Oct;18(8):2357-64. doi: 10.1046/j.1460-9568.2003.02932.x. Eur J Neurosci. 2003. PMID: 14622198 - Corticosterone modulates autonomic responses and adaptation of central immediate-early gene expression to repeated restraint stress.
Stamp J, Herbert J. Stamp J, et al. Neuroscience. 2001;107(3):465-79. doi: 10.1016/s0306-4522(01)00364-5. Neuroscience. 2001. PMID: 11719001 - The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress.
McKlveen JM, Myers B, Herman JP. McKlveen JM, et al. J Neuroendocrinol. 2015 Jun;27(6):446-56. doi: 10.1111/jne.12272. J Neuroendocrinol. 2015. PMID: 25737097 Free PMC article. Review. - "Braking" the Prefrontal Cortex: The Role of Glucocorticoids and Interneurons in Stress Adaptation and Pathology.
McKlveen JM, Moloney RD, Scheimann JR, Myers B, Herman JP. McKlveen JM, et al. Biol Psychiatry. 2019 Nov 1;86(9):669-681. doi: 10.1016/j.biopsych.2019.04.032. Epub 2019 May 9. Biol Psychiatry. 2019. PMID: 31326084 Review.
Cited by
- Neurocircuitry of mood disorders.
Price JL, Drevets WC. Price JL, et al. Neuropsychopharmacology. 2010 Jan;35(1):192-216. doi: 10.1038/npp.2009.104. Neuropsychopharmacology. 2010. PMID: 19693001 Free PMC article. Review. - How Early Experience Shapes Human Development: The Case of Psychosocial Deprivation.
Nelson CA 3rd, Zeanah CH, Fox NA. Nelson CA 3rd, et al. Neural Plast. 2019 Jan 14;2019:1676285. doi: 10.1155/2019/1676285. Neural Plast. 2019. PMID: 30774652 Free PMC article. Review. - The infralimbic mineralocorticoid blockage prevents the stress-induced impairment of aversive memory extinction in rats.
Albernaz-Mariano KA, Demarchi Munhoz C. Albernaz-Mariano KA, et al. Transl Psychiatry. 2022 Aug 24;12(1):343. doi: 10.1038/s41398-022-02118-2. Transl Psychiatry. 2022. PMID: 35999226 Free PMC article. - Childhood Trauma, the HPA Axis and Psychiatric Illnesses: A Targeted Literature Synthesis.
Murphy F, Nasa A, Cullinane D, Raajakesary K, Gazzaz A, Sooknarine V, Haines M, Roman E, Kelly L, O'Neill A, Cannon M, Roddy DW. Murphy F, et al. Front Psychiatry. 2022 May 6;13:748372. doi: 10.3389/fpsyt.2022.748372. eCollection 2022. Front Psychiatry. 2022. PMID: 35599780 Free PMC article. Review.
References
- Adamec RE, Morgan HD. The effect of kindling of different nuclei in the left and right amygdala on anxiety in the rat. Neuroscience. 1994;55:1–12. - PubMed
- Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–350. - PubMed
- Bacon SJ, Smith AD. A monosynaptic pathway from an identified vasomotor centre in the medial prefrontal cortex to an autonomic area in the thoracic spinal cord. Neuroscience. 1993;54:719–728. - PubMed
- Barden N, Reul JM, Holsboer F. Do antidepressants stabilize mood through actions on the hypothalamic-pituitary-adrenocortical system? Trends Neurosci. 1995;18:6–11. - PubMed
- Bhatnagar S, Meaney MJ. Hypothalamic-pituitary-adrenal function in chronic intermittently cold-stressed neonatally handled and nonhandled rats. J Neuroendocrinol. 1995;7:97–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources