The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis - PubMed (original) (raw)
The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis
S A Laporte et al. Proc Natl Acad Sci U S A. 1999.
Abstract
betaarrestins mediate the desensitization of the beta2-adrenergic receptor (beta2AR) and many other G protein-coupled receptors (GPCRs). Additionally, betaarrestins initiate the endocytosis of these receptors via clathrin coated-pits and interact directly with clathrin. Consequently, it has been proposed that betaarrestins serve as clathrin adaptors for the GPCR family by linking these receptors to clathrin lattices. AP-2, the heterotetrameric clathrin adaptor protein, has been demonstrated to mediate the internalization of many types of plasma membrane proteins other than GPCRs. AP-2 interacts with the clathrin heavy chain and cytoplasmic domains of receptors such as those for epidermal growth factor and transferrin. In the present study we demonstrate the formation of an agonist-induced multimeric complex containing a GPCR, betaarrestin 2, and the beta2-adaptin subunit of AP-2. beta2-Adaptin binds betaarrestin 2 in a yeast two-hybrid assay and coimmunoprecipitates with betaarrestins and beta2AR in an agonist-dependent manner in HEK-293 cells. Moreover, beta2-adaptin translocates from the cytosol to the plasma membrane in response to the beta2AR agonist isoproterenol and colocalizes with beta2AR in clathrin-coated pits. Finally, expression of betaarrestin 2 minigene constructs containing the beta2-adaptin interacting region inhibits beta2AR endocytosis. These findings point to a role for AP-2 in GPCR endocytosis, and they suggest that AP-2 functions as a clathrin adaptor for the endocytosis of diverse classes of membrane receptors.
Figures
Figure 1
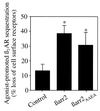
Effect of βarrestins on β2AR sequestration in COS-7 cells. The agonist-mediated sequestration of β2AR was measured in the presence of endogenous βarrestin only (Control), overexpressed βarrestin 2 (βarr2), or overexpressed βarrestin 2AAEA (βarr2AAEA). Equal amounts of βarr2 and βarr2AAEA were expressed (data not shown). Sequestration is defined as the fraction of total cell surface receptors that are not accessible to antibodies after exposure to agonist, and is expressed as percent loss of cell surface receptors. Results demonstrate that βarr2AAEA and wild-type βarr2 expression similarly increase sequestration above control levels (∗, P < 0.01). The data represent the mean ± SD of three or four independent experiments.
Figure 2
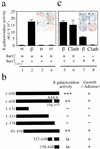
Interaction between βarrestin 2 and individual subunits of AP-2 or clathrin as assessed by β-galactosidase activity in yeast. Results of colony-lift filter assays, colony growth assays on adenine-deficient medium, and liquid culture β-galactosidase assays are shown. (a) The binding interactions between βarrestin 2 (βarr2) and each of the four subunits of AP-2 were analyzed in yeast cells transformed with plasmids encoding GAL4 BD-βarr2 and one of the various GAL4 AD-adaptin subunits (α, β2, μ2, and σ2). (b) Results of the expression of the lacZ reporter gene and adenine growth in yeast cells transformed with GAL4 BD-βarr2, -βarr2AAEA, or different fragments of βarr2 with the GAL4 AD-β2-adaptin subunits. Only transformants expressing interacting proteins were able to grow in the absence of adenine, eliminating the contribution of transformants with intrinsic activity (ia). Positive results for β-galactosidase activity or growth on adenine-deficient plates are indicated by (+), and no interaction is indicated by (−). (c) The interaction of βarr2 or βarrestin2 clathrin-binding deficient mutant (βarr2AAEA) with GAL4 AD-clathrin (Clath) or GAL4 AD-β2-adaptin (β) was examined. A more intense blue coloration on the filter indicates a stronger binding interaction between the two proteins. Results, expressed in relative light units (RLU), are the mean ± SD of triplicate determinations. All results are representative of four to six independent experiments.
Figure 3
β2-Adaptin immunoprecipitation in HEK-293 cells. Cells were transfected with receptor and/or βarrestin 1 (βarr1) or βarrestin 2 (βarr2) cDNA and treated as described in the text. Transfected cells were exposed to agonist for the indicated times, and the cell lysates were immunoprecipitated. (a–c) Amounts of β2-adaptin (arrowheads) that were immunoprecipitated with HA-tagged β2AR and a monoclonal antibody against the HA epitope (a), Flag-tagged βarr1 or βarr2 and a monoclonal antibody against the Flag epitope (b), or HA-tagged μOR and the anti-HA antibody (c). Immunoprecipitation results with agonist-treated mock-transfected cells are shown to the right of each panel for comparison. Results are representative of three to eight experiments.
Figure 4
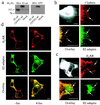
Expression and distribution of GFP/β2-adaptin in HEK-293 cells. (a) Immunoblots of extracts from HEK-293 cells overexpressing β2-adaptin, GFP/β2adaptin, or endogenous β2-adaptin (mock). Proteins were detected with an antibody to β2-adaptin (Left) or GFP (Right). (b) Epifluorescence microscopy of HEK-293 cells shows transfected GFP/β2-adaptin colocalization with clathrin-coated pits. The cell area outlined in Upper Left has been enlarged in the other three panels. Upper Right shows clathrin immunostaining (red), Lower Right shows GFP/β2-adaptin fluorescence (green), and Lower Left shows the overlap (yellow or arrows) obtained from the superimposed clathrin and GFP/β2-adaptin images. (c) Demonstration of colocalization of GFP/β2-adaptin (green, Lower Right) to plasma membrane-bound β2AR (red, Upper Right) after isoproterenol treatment. The cell area outlined in Upper Left has been enlarged in the other three panels. The arrows indicate punctate areas of the plasma membrane where β2AR and β2-adaptin colocalize after isoproterenol treatment. These punctate areas correspond to clathrin-coated pits (5). (d) Confocal images demonstrating the isoproterenol-induced translocation of GFP/β2-adaptin to the plasma membrane. (Top) Plasma membrane distribution of β2AR (red) with (Right) or without (Left) isoproterenol. (Middle) Distribution of GFP/β2-adaptin (green) with or without agonist stimulation. Note the significant increase in plasma membrane localization of GFP/β2-adaptin in the presence of agonist at the expense of the cytosolic signal. In the absence of agonist (Bottom Left), the β2AR signal (red) appears at the plasma membrane, whereas that for the GFP/β2-adaptin (green) is mostly cytosolic. In the presence of agonist (Bottom Right) the fluorescent signals become predominantly coincident at the plasma membrane (yellow). Epifluorescence microscopy was done as previously described (26). Confocal images were obtained on a Zeiss LSM-410 laser scanning confocal microscope. (All scale bars = 10 μm.)
Figure 5
Effect of minigene βarrestin on β2AR sequestration in HEK-293 cells. The agonist-mediated sequestration of β2AR was measured in the presence of endogenous βarrestin only (Control) and βarrestin 2 C-terminal minigene with (βarr2 310–410) or without (βarr2 310–410AAEA) the clathrin-binding site. Similar amounts of βarr2 310–410 and βarr2 310–410AAEA were expressed (data not shown). Results demonstrate that βarr2 310–410 and βarr2 310–410AAEA expression similarly decrease sequestration below control levels (∗, P < 0.01). The data represent the mean ± SD of four independent experiments.
Similar articles
- Src-dependent phosphorylation of beta2-adaptin dissociates the beta-arrestin-AP-2 complex.
Fessart D, Simaan M, Zimmerman B, Comeau J, Hamdan FF, Wiseman PW, Bouvier M, Laporte SA. Fessart D, et al. J Cell Sci. 2007 May 15;120(Pt 10):1723-32. doi: 10.1242/jcs.03444. Epub 2007 Apr 24. J Cell Sci. 2007. PMID: 17456551 - c-Src-mediated phosphorylation of AP-2 reveals a general mechanism for receptors internalizing through the clathrin pathway.
Zimmerman B, Simaan M, Lee MH, Luttrell LM, Laporte SA. Zimmerman B, et al. Cell Signal. 2009 Jan;21(1):103-10. doi: 10.1016/j.cellsig.2008.09.013. Epub 2008 Oct 1. Cell Signal. 2009. PMID: 18938240 - Phosphoinositide 3-kinase regulates beta2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex.
Naga Prasad SV, Laporte SA, Chamberlain D, Caron MG, Barak L, Rockman HA. Naga Prasad SV, et al. J Cell Biol. 2002 Aug 5;158(3):563-75. doi: 10.1083/jcb.200202113. Epub 2002 Aug 5. J Cell Biol. 2002. PMID: 12163475 Free PMC article. - Beta-Arrestins: new roles in regulating heptahelical receptors' functions.
McDonald PH, Lefkowitz RJ. McDonald PH, et al. Cell Signal. 2001 Oct;13(10):683-9. doi: 10.1016/s0898-6568(01)00203-0. Cell Signal. 2001. PMID: 11602178 Review.
Cited by
- G Protein-coupled Receptor Kinases of the GRK4 Protein Subfamily Phosphorylate Inactive G Protein-coupled Receptors (GPCRs).
Li L, Homan KT, Vishnivetskiy SA, Manglik A, Tesmer JJ, Gurevich VV, Gurevich EV. Li L, et al. J Biol Chem. 2015 Apr 24;290(17):10775-90. doi: 10.1074/jbc.M115.644773. Epub 2015 Mar 13. J Biol Chem. 2015. PMID: 25770216 Free PMC article. - Minireview: ubiquitination-regulated G protein-coupled receptor signaling and trafficking.
Alonso V, Friedman PA. Alonso V, et al. Mol Endocrinol. 2013 Apr;27(4):558-72. doi: 10.1210/me.2012-1404. Epub 2013 Mar 7. Mol Endocrinol. 2013. PMID: 23471539 Free PMC article. Review. - The Ubiquitination of Arrestin3 within the Nucleus Triggers the Nuclear Export of Mdm2, Which, in Turn, Mediates the Ubiquitination of GRK2 in the Cytosol.
Kundu D, Min X, Zhang X, Tian X, Wang S, Kim KM. Kundu D, et al. Int J Mol Sci. 2024 Sep 6;25(17):9644. doi: 10.3390/ijms25179644. Int J Mol Sci. 2024. PMID: 39273591 Free PMC article. - Follicle-stimulating hormone (FSH) activates extracellular signal-regulated kinase phosphorylation independently of beta-arrestin- and dynamin-mediated FSH receptor internalization.
Piketty V, Kara E, Guillou F, Reiter E, Crepieux P. Piketty V, et al. Reprod Biol Endocrinol. 2006 Jun 20;4:33. doi: 10.1186/1477-7827-4-33. Reprod Biol Endocrinol. 2006. PMID: 16787538 Free PMC article. - Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor.
Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Mishra SK, et al. EMBO J. 2002 Sep 16;21(18):4915-26. doi: 10.1093/emboj/cdf487. EMBO J. 2002. PMID: 12234931 Free PMC article.
References
- Yu S S, Lefkowitz R J, Hausdorff W P. J Biol Chem. 1993;268:337–341. - PubMed
- Pippig S, Andexinger S, Lohse M J. Mol Pharmacol. 1995;47:666–676. - PubMed
- Ferguson S S, Barak L S, Zhang J, Caron M G. Can J Physiol Pharmacol. 1996;74:1095–1110. - PubMed
- von Zastrow M, Kobilka B K. J Biol Chem. 1992;267:3530–3538. - PubMed
- Zhang J, Ferguson S S G, Barak L S, Menard L, Caron M G. J Biol Chem. 1996;271:18302–18305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous