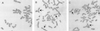Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening - PubMed (original) (raw)
Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening
J Zhu et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Human fibroblasts whose lifespan in culture has been extended by expression of a viral oncogene eventually undergo a growth crisis marked by failure to proliferate. It has been proposed that telomere shortening in these cells is the property that limits their proliferation. Here we report that ectopic expression of the wild-type reverse transcriptase protein (hTERT) of human telomerase averts crisis, at the same time reducing the frequency of dicentric and abnormal chromosomes. Surprisingly, as the resulting immortalized cells containing active telomerase continue to proliferate, their telomeres continue to shorten to mean lengths below those in control cells that enter crisis. These results provide evidence for a protective function of human telomerase that allows cell proliferation without requiring net lengthening of telomeres.
Figures
Figure 1
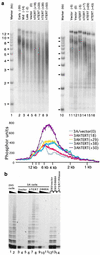
Telomere length and telomerase activity in hTERT-expressing human fibroblasts. (a) Southern analyses of telomere length. Lanes 2–9 are 3A cells and lanes 11–16 are PLR cells. The numbers across the top indicate the relative population doublings at which DNA were made. The stage at which cells entered crisis was designated as population doubling 0. Early, Mid, and Late indicate precrisis cells at 35, 14, and 2 population doublings before they entered crisis, respectively. Vector indicates vector virus-infected cells. hTERT represents the cells transduced with hTERT virus. The numbers on the left are the sizes of DNA markers (kb) (lanes 1 and 10). (Lower) PhosphorImaging scans of telomere hybridization signals in lanes 5–9. (b) Telomerase activity in 3A cells. Telomerase assay was carried out using a modified telomeric repeat amplification protocol, followed by product separation on a 10% denaturing polyacrylamide gel. The triangles across the top indicate the relative amount of protein extracts used in assays. On the left of each set of assays, 2 μl of extract (0.5 μg/μl protein) was used. In the next two assays, 1/20 and 1/400 distributions of extract were used. In 293/vector and 293/D868A lanes, 293 cell extract was mixed with 3A cells extracts (0.5 μg each) transduced with vector and hTERT/D868A viruses. In the hTERT/RNase lane, 0.5 μg of 3A/hTERT cell extract was mixed with RNase.
Figure 2
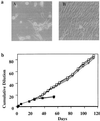
Effects of hTERT expression in human fibroblast cells transformed by SV40 large T antigen. (a) Morphology of 3A cells 4 weeks after they were transduced with either vector (A) or hTERT virus (B). (b) Growth curves of 3A cells infected with either hTERT (open symbols) or vector pBABE viruses (solid symbols). Puromycin-resistant cell pools were grown in 10-cm dishes and passaged when they reached confluence.
Figure 3
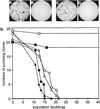
Effect of hTERT expression on cellular proliferation. (a) Colony formation of 3A and PLR cells expressing hTERT. 3A (A and B) and PLR (C and D) cells infected by hTERT virus (A and C) or by vector pBABE virus (B and D) were seeded onto 10-cm dishes at densities of 2,000 cells per 10-cm dish. The colonies were stained with methylene blue 2 weeks after plating. (b) Growth of clonal 3A and PLR cells. 3A (open symbols) and PLR (solid symbols) cells were infected with hTERT (squares), hTERT (D868A) (triangles), or pBABE-puro (circles) viruses, and clonal cells were propagated. The stages at which cells were infected were designated as population 0. When a clone entered crisis, the majority of cells died and the culture was discontinued.
Figure 4
Effect of hTERT expression on dicentric chromosome formation. Metaphase spreads of 3A cells were visualized with Giemsa staining (A and B) or C-band staining (C). A shows hTERT virus-transduced cells and B and C show vector-infected cells. Arrows point to dicentric chromosomes.
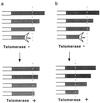
Figure 5
Models for the telomere-capping function(s) of telomerase. (a) Preferential elongation upon hTERT expression of telomeres that are critically short. Dotted lines indicate a proposed threshold of telomere length below which telomeres may be compromised in their function. (b) Telomere protection by telomerase. In the absence of telomerase, telomeres lose their stabilizing function when they are shortened below a threshold length (dashed line). The presence of active telomerase lowers this threshold. Open and shaded portions of the horizontal bars indicate the interior of chromosomes and telomeric sequences, respectively.
Comment in
- Crisis intervention: the role of telomerase.
Lustig AJ. Lustig AJ. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3339-41. doi: 10.1073/pnas.96.7.3339. Proc Natl Acad Sci U S A. 1999. PMID: 10097039 Free PMC article. Review. No abstract available.
References
- Hayflick L, Moorhead P S. Exp Cell Res. 1961;25:585–621. - PubMed
- Shay J W, Pereira-Smith O M, Wright W E. Exp Cell Res. 1991;196:33–39. - PubMed
- Hara E, Tsurui H, Shinozaki A, Nakada S, Oda K. Biochem Biophys Res Commun. 1991;179:528–534. - PubMed
- Niida H, Matsumoto T, Satoh H, Shiwa M, Tokutake Y, Furuichi Y, Shinkai Y. Nat Genet. 1998;19:203–206. - PubMed
- McEachern M J, Blackburn E H. Genes Dev. 1996;10:1822–1834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources