Ontogeny of T cell tolerance to peripherally expressed antigens - PubMed (original) (raw)
Ontogeny of T cell tolerance to peripherally expressed antigens
D J Morgan et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Transgenic expression of the influenza virus hemagglutinin (HA) in the pancreatic islet beta cells of InsHA mice leads to peripheral tolerance of HA-specific T cells. To examine the onset of tolerance, InsHA mice were immunized with influenza virus A/PR/8 at different ages, and the presence of nontolerant T cells was determined by the induction of autoimmune diabetes. The data revealed a neonatal period wherein T cells were not tolerant and influenza virus infection led to HA-specific beta cell destruction and autoimmune diabetes. The ability to induce autoimmunity gradually waned, such that adult mice were profoundly tolerant to viral HA and were protected from diabetes. Because cross-presentation of islet antigens by professional antigen-presenting cells had been reported to induce peripheral tolerance, the temporal relationship between tolerance induction and activation of HA-specific T cells in the lymph nodes draining the pancreas was examined. In tolerant adult mice, but not in 1-week-old neonates, activation and proliferation of HA-specific CD8(+) T cells occurred in the pancreatic lymph nodes. Thus, lack of tolerance in the perinatal period correlated with lack of activation of antigen-specific CD8(+) T cells. This work provides evidence for the developmental regulation of peripheral tolerance induction.
Figures
Figure 1
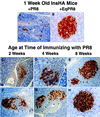
Insulitis in neonatal InsHA mice immunized with PR8. Neonatal InsHA mice at various ages as shown were immunized i.p. with 1,200 HA units of PR8. Shown are immunohistological analyses of pancreata taken from various InsHA mice immunized with PR8. Paraffin-embedded sections are stained for insulin by using the immunoperoxidase technique with diaminobenzidine as a chromagen and counterstained with hematoxylin. (A) Tissue isolated from 1-week-old neonate 9 days after immunization with PR8. Note the overwhelming presence of mononuclear cells, arrows indicate edges of islet remnants leaving only a few insulin positive β cells. (B) One-week-old neonate 9 days after immunization with EqPR8. Islet is free from mononuclear infiltration and uniform insulin staining shows there is no β cell destruction. (C) Two-week-old and (D) 4-week-old mice 9 days after immunizing with PR8, both show considerable insulitis and β cell destruction (arrows). Mice from the same groups, 2 weeks old (F) and 4 weeks old (G), 21 days after immunization. The extent of insulitis and β cell destruction is less at this time (arrows). (E) Representative of the most severe insulitis demonstrated in 8-week-old adult mice 9 days after immunization with PR8. Only a mild peri-insulitis is observed that is not associated with any β cell destruction (arrows). (H) Representative of >90% of islets from these same adults 21 days after immunization. They are intact and express high levels of insulin. Magnifications: A, ×100; B, D, and H, ×400; C and E_–_G, ×200.
Figure 2
CSFE-labeled, purified clone-4 TCR CD8+ T cells do not proliferate in the pancreatic lymph nodes after adoptive transfer into neonatal InsHA mice. Adult InsHA negative (A_–_C) and adult InsHA positive (D_–_F) mice were injected i.v., and 5-day-old neonatal InsHA mice (G and H) were injected i.p. with 5 × 106 CSFE-labeled, purified clone-4 TCR CD8+ T cells. Seventy-two hours later cells isolated from various peripheral lymphoid organs as shown were stained with phycoerythrin-conjugated anti-CD8 antibodies. Data show amount of CSFE label among activated CSFE-labeled CD8+ T cells obtained from pooled lymphoid tissue taken from at least three adult mice per group and seven neonates. For clarity of depiction, the unlabeled cells have been deleted from the histograms.
Similar articles
- Defective CD8+ T cell peripheral tolerance in nonobese diabetic mice.
Kreuwel HT, Biggs JA, Pilip IM, Pamer EG, Lo D, Sherman LA. Kreuwel HT, et al. J Immunol. 2001 Jul 15;167(2):1112-7. doi: 10.4049/jimmunol.167.2.1112. J Immunol. 2001. PMID: 11441123 - CD8(+) T cells specific for beta cells encounter their cognate antigens in the islets of NOD mice.
Pang S, Zhang L, Wang H, Yi Z, Li L, Gao L, Zhao J, Tisch R, Katz JD, Wang B. Pang S, et al. Eur J Immunol. 2009 Oct;39(10):2716-24. doi: 10.1002/eji.200939408. Eur J Immunol. 2009. PMID: 19658094 - The TCR-HA, INS-HA transgenic model of autoimmune diabetes: limitations and expectations.
Apostolou I, Von Boehmer H. Apostolou I, et al. J Autoimmun. 2004 Mar;22(2):111-4. doi: 10.1016/j.jaut.2003.10.005. J Autoimmun. 2004. PMID: 14987738 Review. No abstract available. - T-cell receptor transgenic (TCR-Tg) mice from two diabetogenic CD4+ islet-antigen-specific T-cell clones.
Haskins K. Haskins K. J Autoimmun. 2004 Mar;22(2):107-9. doi: 10.1016/j.jaut.2003.10.006. J Autoimmun. 2004. PMID: 14987737 Review. No abstract available.
Cited by
- TCR Transgenic Mice: A Valuable Tool for Studying Viral Immunopathogenesis Mechanisms.
Cho YB, Lee IG, Joo YH, Hong SH, Seo YJ. Cho YB, et al. Int J Mol Sci. 2020 Dec 18;21(24):9690. doi: 10.3390/ijms21249690. Int J Mol Sci. 2020. PMID: 33353154 Free PMC article. Review. - Revisiting T Cell Tolerance as a Checkpoint Target for Cancer Immunotherapy.
Nüssing S, Trapani JA, Parish IA. Nüssing S, et al. Front Immunol. 2020 Sep 23;11:589641. doi: 10.3389/fimmu.2020.589641. eCollection 2020. Front Immunol. 2020. PMID: 33072137 Free PMC article. Review. - Peripheral Deletion of CD8 T Cells Requires p38 MAPK in Cross-Presenting Dendritic Cells.
Smith T, Lin X, Mello M, Marquardt K, Cheung J, Lu B, Sherman LA, Verdeil G. Smith T, et al. J Immunol. 2017 Oct 15;199(8):2713-2720. doi: 10.4049/jimmunol.1700427. Epub 2017 Sep 1. J Immunol. 2017. PMID: 28864471 Free PMC article. - Low Antigen Dose in Adjuvant-Based Vaccination Selectively Induces CD4 T Cells with Enhanced Functional Avidity and Protective Efficacy.
Billeskov R, Wang Y, Solaymani-Mohammadi S, Frey B, Kulkarni S, Andersen P, Agger EM, Sui Y, Berzofsky JA. Billeskov R, et al. J Immunol. 2017 May 1;198(9):3494-3506. doi: 10.4049/jimmunol.1600965. Epub 2017 Mar 27. J Immunol. 2017. PMID: 28348274 Free PMC article. - A replicating modified vaccinia tiantan strain expressing an avian-derived influenza H5N1 hemagglutinin induce broadly neutralizing antibodies and cross-clade protective immunity in mice.
Xiao H, Liu L, Zhu Q, Tan Z, Yu W, Tang X, Zhan D, Du Y, Wang H, Liu D, Li Z, Yuen KY, Ho DD, Gao GF, Chen Z. Xiao H, et al. PLoS One. 2013 Dec 17;8(12):e83274. doi: 10.1371/journal.pone.0083274. eCollection 2013. PLoS One. 2013. PMID: 24358269 Free PMC article.
References
- Kappler J W, Roehm N, Marrack P. Cell. 1987;49:273–280. - PubMed
- Kisielow P, Bluthmann H, Staerz U D, Steinmetz M, von Boehmer H. Nature (London) 1988;333:742–746. - PubMed
- Sprent J, Schaefer M. Immunol Rev. 1990;117:213–234. - PubMed
- Webb S R, Sprent J. Science. 1990;248:1643–1646. - PubMed
- Webb S, Morris C, Sprent J. Cell. 1990;63:1249–1256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials