Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier - PubMed (original) (raw)
Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier
V V Rao et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The blood-brain barrier and a blood-cerebrospinal-fluid (CSF) barrier function together to isolate the brain from circulating drugs, toxins, and xenobiotics. The blood-CSF drug-permeability barrier is localized to the epithelium of the choroid plexus (CP). However, the molecular mechanisms regulating drug permeability across the CP epithelium are defined poorly. Herein, we describe a drug-permeability barrier in human and rodent CP mediated by epithelial-specific expression of the MDR1 (multidrug resistance) P glycoprotein (Pgp) and the multidrug resistance-associated protein (MRP). Noninvasive single-photon-emission computed tomography with 99mTc-sestamibi, a membrane-permeant radiopharmaceutical whose transport is mediated by both Pgp and MRP, shows a large blood-to-CSF concentration gradient across intact CP epithelium in humans in vivo. In rats, pharmacokinetic analysis with 99mTc-sestamibi determined the concentration gradient to be greater than 100-fold. In membrane fractions of isolated native CP from rat, mouse, and human, the 170-kDa Pgp and 190-kDa MRP are identified readily. Furthermore, the murine proteins are absent in CP isolated from their respective mdr1a/1b(-/-) and mrp(-/-) gene knockout littermates. As determined by immunohistochemical and drug-transport analysis of native CP and polarized epithelial cell cultures derived from neonatal rat CP, Pgp localizes subapically, conferring an apical-to-basal transepithelial permeation barrier to radiolabeled drugs. Conversely, MRP localizes basolaterally, conferring an opposing basal-to-apical drug-permeation barrier. Together, these transporters may coordinate secretion and reabsorption of natural product substrates and therapeutic drugs, including chemotherapeutic agents, antipsychotics, and HIV protease inhibitors, into and out of the central nervous system.
Figures
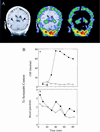
Figure 1
Detection of B-CSF permeability barrier for 99mTc-sestamibi in vivo in human and rat. (A) Coronal human brain SPECT image 90 min after intravenous injection of 99mTc-sestamibi (Right); a gadolinium-chelate contrast-enhanced T1-weighted coronal head MRI (Left); and coregistered image (Center) of a human volunteer. Arrowheads on the MRI demarcate CP visualized bilaterally within the lateral ventricles. Note the radioactive drug localized to the CP. (B) In anesthetized rats, microdialysate samples from a cannulated lateral ventricle (○, ●) and concurrent blood samples from the periorbital plexus (□, ■) were collected at the indicated times after tail-vein injection of 99mTc-sestamibi in the absence (○, □) or presence of 250 mg/kg GF120918 (●, ■) and then counted for γ-activity. In the absence of GF120918, there is a 100-fold lower drug concentration in CSF compared with blood. Data represent a typical experiment from three independent experiments under each condition.
Figure 2
(A) Expression of Pgp and MRP (arrows) in enriched membrane fractions of CP from rat (rCP) and human (hCP) shown by immunoblotting with C219 (a), MRPr1 (b), and QCRL-1 (c). Disruption of Pgp and MRP expression in cell lysates of CP isolated from FVB mdr1a/1b(−/−) and C57Bl/6 mrp(−/−) gene knockout mice, respectively, compared with their wild-type littermates shown by immunoblotting with C219 (d) and MRPr1 (e). C219 detects a protein of 170 kDa that comigrates with MDR1 Pgp expressed in human multidrug-resistant KB 8-5 cells, whereas MRPr1 and QCRL-1 detect a protein of 190 kDa that comigrates with recombinant human MRP expressed in NIH 3T3 fibroblasts. In all lanes 50 μg of protein was used, except for NIH 3T3 MRP (25 μg). (B) Localization of Pgp to epithelial cells of rat and human CP by immunohistochemistry. Frozen rat brain tissue is stained in the absence of primary mAb (a; ×100), with mAb C219 (b; ×100), or with C219 (c; ×400); staining is abolished competitively by preequilibrating C219 in a 1,000-fold molar excess of a synthetic epitope-specific blocking peptide (d; VVQEALDKAREGRTC; ×400). Frozen human CP is stained in the absence of primary mAb (e; ×400) and in the presence of mAb MRK16 (f; ×400). Human CP epithelial cell-specific expression also was confirmed by peptide-displaceable staining with C219 (data not shown). (b) cp, CP within a lateral ventricle; the arrowhead indicates expression of Pgp in the capillary endothelial cells of rat brain parenchyma. (C) Localization of MRP to epithelial cells of rat and human CP by immunohistochemistry. Rat-brain sections immunostained in the absence of primary mAb (a; ×100) and in the presence of mAb MRPr1 (b; ×100). Human CP tissue stained with mAb MRPr1 (c; ×400) and mAb QCRL-1 (d; ×400).
Figure 3
Conventional electron microscopy of monolayers of cultured neonatal rat CP epithelial cells. Note abundant apical microvilli on the apical surface (A), the formation of basement membrane adjacent to the filter, and desmosomes between cells near their apical surfaces (B), features characteristic of native CP epithelia in vivo (18). MV, microvilli; B, basement membrane; N, nucleus; J, desmosome/tight junction complex. (Bar = 0.5 μm.)
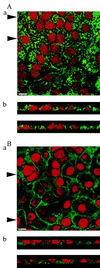
Figure 4
Localization of Pgp expression with mAb C219 (A) and MRP expression with mAb MRPr1 (B) in cultured neonatal rat CP epithelial cells by confocal microscopy. (Aa and Ba) Top view of the cell layer. (Bar = 10 μm.) (Ab and Bb) Optical sections perpendicular to the plane of the cell layer. Arrowheads in Aa and Ba indicate positions of the respective sections in Ab and Bb. Pgp localizes predominantly toward apical surfaces, whereas MRP localizes toward basolateral surfaces.
Figure 5
Transepithelial transport of 99mTc-sestamibi and [3H]Taxol across confluent monolayers of rat CP epithelial cells, wherein drug is added to either the basal (Aa, Ba, and Ca) or the apical (Ab, Bb, and Cb) side of the monolayers. (A) 99mTc-sestamibi added in the absence and presence of GF120918 (300 nM). (B) 99mTc-sestamibi added in the absence and presence of MK-571 (100 μM). (C) [3H]Taxol added in the absence and presence of GF120918 (300 nM). Each point represents the mean value of three monolayers; bars represent ±SEM when larger than the symbol.
Similar articles
- Effects of MDR1 and MDR3 P-glycoproteins, MRP1, and BCRP/MXR/ABCP on the transport of (99m)Tc-tetrofosmin.
Chen WS, Luker KE, Dahlheimer JL, Pica CM, Luker GD, Piwnica-Worms D. Chen WS, et al. Biochem Pharmacol. 2000 Aug 1;60(3):413-26. doi: 10.1016/s0006-2952(00)00341-5. Biochem Pharmacol. 2000. PMID: 10856437 - Drug resistance caused by multidrug resistance-associated proteins.
Wijnholds J. Wijnholds J. Novartis Found Symp. 2002;243:69-79; discussion 80-2, 180-5. Novartis Found Symp. 2002. PMID: 11990783 Review. - Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation.
Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. Roberts LM, et al. Neuroscience. 2008 Aug 13;155(2):423-38. doi: 10.1016/j.neuroscience.2008.06.015. Epub 2008 Jun 13. Neuroscience. 2008. PMID: 18619525 - Brain drug delivery, drug metabolism, and multidrug resistance at the choroid plexus.
Ghersi-Egea JF, Strazielle N. Ghersi-Egea JF, et al. Microsc Res Tech. 2001 Jan 1;52(1):83-8. doi: 10.1002/1097-0029(20010101)52:1<83::AID-JEMT10>3.0.CO;2-N. Microsc Res Tech. 2001. PMID: 11135451 Review.
Cited by
- Linkage disequilibrium between polymorphisms of ABCB1 and ABCC2 to predict the treatment outcome of Malaysians with complex partial seizures on treatment with carbamazepine mono-therapy at the Kuala Lumpur Hospital.
Subenthiran S, Abdullah NR, Joseph JP, Muniandy PK, Mok BT, Kee CC, Ismail Z, Mohamed Z. Subenthiran S, et al. PLoS One. 2013 May 23;8(5):e64827. doi: 10.1371/journal.pone.0064827. Print 2013. PLoS One. 2013. PMID: 23717663 Free PMC article. - Characterization of efflux transport proteins of the human choroid plexus papilloma cell line HIBCPP, a functional in vitro model of the blood-cerebrospinal fluid barrier.
Bernd A, Ott M, Ishikawa H, Schroten H, Schwerk C, Fricker G. Bernd A, et al. Pharm Res. 2015 Sep;32(9):2973-82. doi: 10.1007/s11095-015-1679-1. Epub 2015 May 19. Pharm Res. 2015. PMID: 25986174 - Expression of multidrug resistance-associated protein (MRP) in human retinal pigment epithelial cells and its interaction with BAPSG, a novel aldose reductase inhibitor.
Aukunuru JV, Sunkara G, Bandi N, Thoreson WB, Kompella UB. Aukunuru JV, et al. Pharm Res. 2001 May;18(5):565-72. doi: 10.1023/a:1011060705599. Pharm Res. 2001. PMID: 11465409 - Cyclosporine A (CsA) affects the pharmacodynamics and pharmacokinetics of the atypical antipsychotic amisulpride probably via inhibition of P-glycoprotein (P-gp).
Schmitt U, Abou El-Ela A, Guo LJ, Glavinas H, Krajcsi P, Baron JM, Tillmann C, Hiemke C, Langguth P, Härtter S. Schmitt U, et al. J Neural Transm (Vienna). 2006 Jul;113(7):787-801. doi: 10.1007/s00702-005-0367-4. Epub 2005 Oct 27. J Neural Transm (Vienna). 2006. PMID: 16252067 - PRESTA: associating promoter sequences with information on gene expression.
Mach V. Mach V. Genome Biol. 2002 Aug 21;3(9):research0050. doi: 10.1186/gb-2002-3-9-research0050. Epub 2002 Aug 21. Genome Biol. 2002. PMID: 12225589 Free PMC article.
References
- Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. - PubMed
- Cole S P C, Bhardwaj G, Gerlach J H, Mackie J E, Grant C E, Almquist K C, Stewart A J, Kurz E U, Duncan A M V, Deeley R G. Science. 1992;258:1650–1654. - PubMed
- Zaman G J R, Versantvoort C H M, Smit J J M, Eijdems W H M, de Haas M, Smith A J, Broxterman H J, Mulder N H, de Vries E G E, Baas F, et al. Cancer Res. 1993;53:1747–1750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous