The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus-mediated adhesion - PubMed (original) (raw)
The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus-mediated adhesion
C Pujol et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The ability of Neisseria meningitidis (MC) to interact with cellular barriers is essential to its pathogenesis. With epithelial cells, this process has been modeled in two steps. The initial stage of localized adherence is mediated by bacterial pili. After this phase, MC disperse and lose piliation, thus leading to a diffuse adherence. At this stage, microvilli have disappeared, and MC interact intimately with cells and are, in places, located on pedestals of actin, thus realizing attaching and effacing (AE) lesions. The bacterial attributes responsible for these latter phenotypes remain unidentified. Considering that bacteria are nonpiliated at this stage, pili cannot be directly responsible for this effect. However, the initial phase of pilus-mediated localized adherence is required for the occurrence of diffuse adherence, loss of microvilli, and intimate attachment, because nonpiliated bacteria are not capable of such a cellular interaction. In this work, we engineered a mutation in the cytoplasmic nucleotide-binding protein PilT and showed that this mutation increased piliation and abolished the dispersal phase of bacterial clumps as well as the loss of piliation. Furthermore, no intimate attachment nor AE lesions were observed. On the other hand, PilT- MC remained adherent as piliated clumps at all times. Taken together these data demonstrate that the induction of diffuse adherence, intimate attachment, and AE lesions after pilus-mediated adhesion requires the cytoplasmic PilT protein.
Figures
Figure 1
Western blot of total bacterial extracts showing electrophoretic migration of clone12 (1), clone12_pilT_− (2), and clone12pilT+ (3). Total bacterial extracts were run in SDS/PAGE, 12% acrylamide, and 3 M urea and transferred to nitrocellulose. The 13C5 mAb (generous gift of L. Brossay), which is directed against the PilT protein of N. gonorrhoeae, was used for PilT detection.
Figure 2
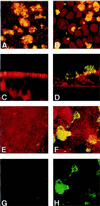
SEM examination of a T84 monolayer infected for 4 h (A_–_D) or 9 h (E_–_L) by PilT+ (A, B, E, F, and I) or PilT− bacteria (C, D, G, H, J, K, and L). Initially, both strains have a similar pattern of adhesion (A_–_D); however, after a longer incubation period, PilT+ spread onto the monolayer (E and F) and adhere very intimately to cells (I). In contrast, PilT− bacteria remain as large, piliated clumps sitting on the cells (G, H, J, K, and L) (Bars = 2 μm.)
Figure 3
Confocal immunofluorescence micrographs of T84 cells grown on coverslips infected for 4 (A and B) or 9 (C_–_H) h by clone12_pilT_+ (A, C, E, and G) or by clone12_pilT_− (B, D, F, and H). MC and eukaryotic cells were stained with ethidium bromide (A, B, C, E, and G) or with tetramethylrhodamine B isothiocyanate-labeled lectin from Triticum vulgaris (D, F, and H) (shown in red). Pili were labeled using the 20D9 mAb (shown in green). A_–_F corresponds to the superimposition of both stainings. G and H display only pili staining of adherent MC. A, B, E, F, G, and H are images reconstructed from confocal xy sections of infected monolayers, and C and D are from xz sections.
Figure 4
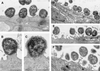
Transmission electron micrographs of T84 cells grown on transwells, infected by PilT+ (_A_– C) or PilT− bacteria (_D_– F) during 9 h. PilT− bacteria adhere to the epithelial cells without ever inducing intimate attachment even though they were in close contact with the cytoplasmic membrane of T84 cells (arrows). Note the presence of numerous microvilli at the surface of epithelial cells (_D_– F) (Bars = 0.5 μm.)
Similar articles
- Pilus-mediated adhesion of Neisseria meningitidis is negatively controlled by the pilus-retraction machinery.
Yasukawa K, Martin P, Tinsley CR, Nassif X. Yasukawa K, et al. Mol Microbiol. 2006 Jan;59(2):579-89. doi: 10.1111/j.1365-2958.2005.04954.x. Mol Microbiol. 2006. PMID: 16390451 - Neisseria PilC protein identified as type-4 pilus tip-located adhesin.
Rudel T, Scheurerpflug I, Meyer TF. Rudel T, et al. Nature. 1995 Jan 26;373(6512):357-9. doi: 10.1038/373357a0. Nature. 1995. PMID: 7830772 - Type-4 pili and meningococcal adhesiveness.
Nassif X, Marceau M, Pujol C, Pron B, Beretti JL, Taha MK. Nassif X, et al. Gene. 1997 Jun 11;192(1):149-53. doi: 10.1016/s0378-1119(96)00802-5. Gene. 1997. PMID: 9224885 Review. - Interaction of pathogenic neisseriae with nonphagocytic cells.
Nassif X, So M. Nassif X, et al. Clin Microbiol Rev. 1995 Jul;8(3):376-88. doi: 10.1128/CMR.8.3.376. Clin Microbiol Rev. 1995. PMID: 7553571 Free PMC article. Review.
Cited by
- Modulation of Kingella kingae adherence to human epithelial cells by type IV Pili, capsule, and a novel trimeric autotransporter.
Porsch EA, Kehl-Fie TE, St Geme JW 3rd. Porsch EA, et al. mBio. 2012 Oct 23;3(5):e00372-12. doi: 10.1128/mBio.00372-12. mBio. 2012. PMID: 23093386 Free PMC article. - The REP2 repeats of the genome of Neisseria meningitidis are associated with genes coordinately regulated during bacterial cell interaction.
Morelle S, Carbonnelle E, Nassif X. Morelle S, et al. J Bacteriol. 2003 Apr;185(8):2618-27. doi: 10.1128/JB.185.8.2618-2627.2003. J Bacteriol. 2003. PMID: 12670987 Free PMC article. - Host cell-derived lactate functions as an effector molecule in Neisseria meningitidis microcolony dispersal.
Sigurlásdóttir S, Engman J, Eriksson OS, Saroj SD, Zguna N, Lloris-Garcerá P, Ilag LL, Jonsson AB. Sigurlásdóttir S, et al. PLoS Pathog. 2017 Apr 6;13(4):e1006251. doi: 10.1371/journal.ppat.1006251. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28384279 Free PMC article. - Function and Benefits of Natural Competence in Cyanobacteria: From Ecology to Targeted Manipulation.
Schirmacher AM, Hanamghar SS, Zedler JAZ. Schirmacher AM, et al. Life (Basel). 2020 Oct 22;10(11):249. doi: 10.3390/life10110249. Life (Basel). 2020. PMID: 33105681 Free PMC article. Review. - PilT and PilU are homohexameric ATPases that coordinate to retract type IVa pili.
Chlebek JL, Hughes HQ, Ratkiewicz AS, Rayyan R, Wang JC, Herrin BE, Dalia TN, Biais N, Dalia AB. Chlebek JL, et al. PLoS Genet. 2019 Oct 18;15(10):e1008448. doi: 10.1371/journal.pgen.1008448. eCollection 2019 Oct. PLoS Genet. 2019. PMID: 31626631 Free PMC article.
References
- Girón J A, Suk Yue Ho A, Schoolnik G K. Science. 1991;254:710–713. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources