Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells - PubMed (original) (raw)
Comparative Study
Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells
M Bhatia et al. J Exp Med. 1999.
Abstract
The identification of molecules that regulate human hematopoietic stem cells has focused mainly on cytokines, of which very few are known to act directly on stem cells. Recent studies in lower organisms and the mouse have suggested that bone morphogenetic proteins (BMPs) may play a critical role in the specification of hematopoietic tissue from the mesodermal germ layer. Here we report that BMPs regulate the proliferation and differentiation of highly purified primitive human hematopoietic cells from adult and neonatal sources. Populations of rare CD34(+)CD38(-)Lin- stem cells were isolated from human hematopoietic tissue and were found to express the BMP type I receptors activin-like kinase (ALK)-3 and ALK-6, and their downstream transducers SMAD-1, -4, and -5. Treatment of isolated stem cell populations with soluble BMP-2, -4, and -7 induced dose-dependent changes in proliferation, clonogenicity, cell surface phenotype, and multilineage repopulation capacity after transplantation in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Similar to transforming growth factor beta, treatment of purified cells with BMP-2 or -7 at high concentrations inhibited proliferation yet maintained the primitive CD34(+)CD38(-) phenotype and repopulation capacity. In contrast, low concentrations of BMP-4 induced proliferation and differentiation of CD34(+) CD38(-)Lin- cells, whereas at higher concentrations BMP-4 extended the length of time that repopulation capacity could be maintained in ex vivo culture, indicating a direct effect on stem cell survival. The discovery that BMPs are capable of regulating repopulating cells provides a new pathway for controlling human stem cell development and a powerful model system for studying the biological mechanism of BMP action using primary human cells.
Figures
Figure 1
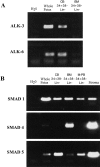
Expression of BMP receptors and SMADs in primitive hematopoietic tissue derived from human sources. RT-PCR reactions were performed on human CD34+CD38−Lin− cells from CB (n = 4), BM (n = 2), M-PB (n = 2), and stromal cells (n = 2) as indicated for (A) BMP receptors ALK-3 and -6, and (B) human SMAD-1, -4, and -5. RT-PCR was performed on whole human fetus sample as a positive control for the reaction.
Figure 2
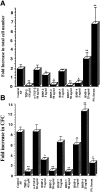
Effect of ex vivo culture on the total cell number and number of clonogenic progenitors present after in vitro culture of CD34+CD38−Lin− cells in the presence of BMPs. (A) Purified CD34+CD38−Lin− cells were counted and seeded (700–1,000) in wells containing serum-free media or with the addition of factors indicated at day 0. Cells were harvested from individual wells after 3 d of culture and counted, and the mean fold increase in absolute cell number was calculated (n = 4). (B) An aliquot of 100–300 CD34+CD38−Lin− cells was plated in progenitor cell assays at the initiation of ex vivo cultures (day 0), and the frequency of progenitors was calculated. Similar cell doses were plated from wells harvested after 3 d of cultures containing the various factors indicated, and the mean fold increase in number of CFCs was calculated compared with day 0 (n = 3). Values are the mean ± SEM of determinations in four and three separate culture samples for cell number and clonogenic progenitors, respectively. *P < 0.05, **P < 0.01 indicate statistically significant differences from controls.
Figure 3
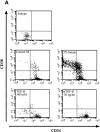
Comparative analysis of CD34 and CD38 expression of highly purified CD34+CD38−Lin− cells after 4 d of culture in the presence of BMPs. A representative experiment (n = 3) of CD34 and CD38 cell surface expression performed on initially purified CD34+CD38−Lin− cells after 4 d of culture in serum-free conditions or with the addition of factors as indicated. The entire contents of individual wells were collected at 4 d, stained with mAbs, and analyzed using flow cytometric analysis.
Figure 3
Comparative analysis of CD34 and CD38 expression of highly purified CD34+CD38−Lin− cells after 4 d of culture in the presence of BMPs. A representative experiment (n = 3) of CD34 and CD38 cell surface expression performed on initially purified CD34+CD38−Lin− cells after 4 d of culture in serum-free conditions or with the addition of factors as indicated. The entire contents of individual wells were collected at 4 d, stained with mAbs, and analyzed using flow cytometric analysis.
Figure 4
Analysis of CD34 and CD38 expression of highly purified CD34+ CD38−Lin− cells after 6 d of culture in the presence of BMP-4. A representative experiment (n = 3) of CD34 and CD38 cell surface expression performed on initially purified CD34+CD38−Lin− cells after 6 d of culture in serum-free conditions or with the addition of BMP-4 at 5 or 25 ng/ml. The entire contents of individual wells were collected at 6 d, stained with mAbs, and analyzed using flow cytometric analysis.
Similar articles
- Bone morphogenetic proteins.
Chen D, Zhao M, Mundy GR. Chen D, et al. Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review. - BMP-7 improved proliferation and hematopoietic reconstitution potential of ex vivo expanded cord blood-derived CD34(+) cells.
Su YH, Cai HB, Ye ZY, Tan WS. Su YH, et al. Hum Cell. 2015 Jan;28(1):14-21. doi: 10.1007/s13577-014-0098-7. Epub 2014 Sep 6. Hum Cell. 2015. PMID: 25192984 - Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity.
Hess DA, Meyerrose TE, Wirthlin L, Craft TP, Herrbrich PE, Creer MH, Nolta JA. Hess DA, et al. Blood. 2004 Sep 15;104(6):1648-55. doi: 10.1182/blood-2004-02-0448. Epub 2004 Jun 3. Blood. 2004. PMID: 15178579 - Characterization of human hematopoietic cells with short-lived in vivo repopulating activity.
Eaves C, Glimm H, Eisterer W, Audet J, Maguer-Satta V, Piret J. Eaves C, et al. Ann N Y Acad Sci. 2001 Jun;938:63-70; discussion 70-1. doi: 10.1111/j.1749-6632.2001.tb03575.x. Ann N Y Acad Sci. 2001. PMID: 11458527 Review.
Cited by
- The effect of bone morphogenetic protein 4 on the differentiation of mouse embryonic stem cell to erythroid lineage in serum free and serum supplemented media.
Ali Owchi M, Salehnia M, Moghadam MF, Boroujeni MB, Hajizadeh E. Ali Owchi M, et al. Int J Biomed Sci. 2009 Sep;5(3):275-82. Int J Biomed Sci. 2009. PMID: 23675148 Free PMC article. - High BMP4 expression in low/intermediate risk BCP-ALL identifies children with poor outcomes.
Fernández-Sevilla LM, Valencia J, Ortiz-Sánchez P, Fraile-Ramos A, Zuluaga P, Jiménez E, Sacedón R, Martínez-Sánchez MV, Jazbec J, Debeljak M, Fedders B, Stanulla M, Schewe D, Cario G, Minguela A, Ramírez M, Varas A, Vicente Á. Fernández-Sevilla LM, et al. Blood. 2022 Jun 2;139(22):3303-3313. doi: 10.1182/blood.2021013506. Blood. 2022. PMID: 35313334 Free PMC article. - Inhibition of MicroRNA-221 and 222 Enhances Hematopoietic Differentiation from Human Pluripotent Stem Cells via c-KIT Upregulation.
Lee JY, Kim M, Heo HR, Ha KS, Han ET, Park WS, Yang SR, Hong SH. Lee JY, et al. Mol Cells. 2018 Nov 30;41(11):971-978. doi: 10.14348/molcells.2018.0244. Epub 2018 Nov 1. Mol Cells. 2018. PMID: 30396237 Free PMC article. - Altered BMP2/4 Signaling in Stem Cells and Their Niche: Different Cancers but Similar Mechanisms, the Example of Myeloid Leukemia and Breast Cancer.
Guyot B, Lefort S, Voeltzel T, Pécheur EI, Maguer-Satta V. Guyot B, et al. Front Cell Dev Biol. 2022 Jan 3;9:787989. doi: 10.3389/fcell.2021.787989. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35047500 Free PMC article. Review. - TGF-β Family Signaling in Embryonic and Somatic Stem-Cell Renewal and Differentiation.
Mullen AC, Wrana JL. Mullen AC, et al. Cold Spring Harb Perspect Biol. 2017 Jul 5;9(7):a022186. doi: 10.1101/cshperspect.a022186. Cold Spring Harb Perspect Biol. 2017. PMID: 28108485 Free PMC article. Review.
References
- Metcalf D. Hematopoietic regulators: redundancy or subtlety? . Blood. 1993;82:3515–3523. - PubMed
- Metcalf D. Lineage commitment and maturation in hematopoietic cells: the case for extrinsic regulation. Blood. 1998;92:345–347. - PubMed
- Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81:2844–2853. - PubMed
- Huber TL, Zon LI. Transcriptional regulation of blood formation during Xenopusdevelopment. Semin Immunol. 1998;10:103–109. - PubMed
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development (Camb) 1998;125:725–732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials