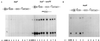Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae - PubMed (original) (raw)
Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae
R R Yu et al. J Bacteriol. 1999 Apr.
Abstract
Coordinate expression of many virulence genes in the human pathogen Vibrio cholerae is controlled by the ToxR, TcpP, and ToxT proteins. These proteins function in a regulatory cascade in which ToxR and TcpP, two inner membrane proteins, are required to activate toxT and ToxT is the direct activator of virulence gene expression. ToxT-activated genes include those whose products are required for the biogenesis of cholera toxin (CTX) and the toxin-coregulated pilus, the major subunit of which is TcpA. This work examined control of toxT transcription. We tested a model whereby activation of toxT by ToxR and TcpP is required to prime an autoregulatory loop in which ToxT-dependent transcription of the tcpA promoter reads through a proposed terminator between the tcpF and toxT genes to result in continued ToxT production. Primer extension analysis of RNA from wild-type classical strain O395 showed that there are two products encoding toxT, one of which is longer than the other by 105 bp. Deletion of the toxT promoter (toxTDeltapro) resulted in the abolishment of toxT transcription, as predicted. Deletion of the tcpA promoter (tcpADeltapro) had no effect on subsequent detection of the smaller toxT primer extension product, but the larger toxT product was not detected, indicating that this product may be the result of transcription from the tcpA promoter and not of initiation directly upstream of toxT. Neither mutant strain produced detectable TcpA, but the CTX levels of the strains were different. The toxTDeltapro strain produced little detectable CTX, while the tcpADeltapro strain produced CTX levels intermediate between those of the wild-type and toxTDeltapro strains. Dependence of toxT transcription on TcpP and TcpH was confirmed by analyzing RNAs from strains carrying deletions in the genes encoding these regulators. The tcpP defect resulted in undetectable toxT transcription, whereas the tcpH mutation led to a diminishing of toxT RNA but not complete abolishment. Taken together, these results suggest that toxT transcription is dependent on two different promoters; one is directly upstream and is activated in part by TcpP and TcpH, and the other is much further upstream and is activated by ToxT.
Figures
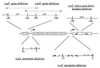
FIG. 1
Schematic diagram of the V. cholerae tcp gene cluster. Shown in the center is the organization of the tcp gene cluster of wild-type V. cholerae. Each box represents a gene. The right-angle arrows represent promoters. Certain regions are enlarged to show the details of each deletion. Brackets indicate the deleted sequence, and the number below each bracket is the nucleotide position, relative to the +1 start site for transcription, which represents the start or the end of a deletion. Arrows at the toxT promoter region denote three pairs of inverted repeats. tcpP and tcpH are in an operon, and their ORFs overlap. The ORFs are shown above the operon. The leftward-pointing arrows beneath toxT and tcpA represent primers used in the RNA primer extension analyses. SD, Shine-Dalgarno region; HTH, helix-turn-helix motif.
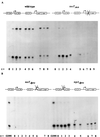
FIG. 2
RNA primer extension analyses of V. cholerae wild-type O395 and a toxTΔhth mutant strain (A) and toxTΔpro and tcpAΔpro mutant strains (B). Samples were collected at 1-h intervals after back dilution of an overnight culture grown in LB medium at 30°C 1:100 into fresh LB medium. A radiolabeled toxT primer was used. RNA collected from wild-type strain O395 after 2 to 3 h of growth was used as a positive control for the mutant strains in panel B.
FIG. 3
RNA primer extension analyses of V. cholerae strain O395 wild-type (A) and mutant strains toxTΔpro and tcpAΔpro (B). Samples were collected at 1-h intervals after back dilution of an overnight culture grown in LB medium at 30°C 1:100 into fresh LB medium. A radiolabeled tcpA primer was used. RNA collected from wild-type strain O395 after 2 to 3 h of growth was used as a positive control for the mutant strains in panel B. The position of the tcpA transcript is indicated.
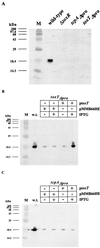
FIG. 4
Western blot analysis using anti-TcpA antibody. V. cholerae wild-type O395 and mutant strains Δ_toxR_, toxTΔpro, and tcpAΔpro (A); wild-type O395 and mutant strain toxTΔpro/pMMB66HE or toxTΔpro/p_toxT_ (B); and wild-type O395 and mutant strain tcpAΔpro/pMMB66HE or tcpAΔpro/p_toxT_ (C) were grown overnight in LB medium at 30°C. IPTG (1 mM) was added to induce toxT transcription in strains carrying plasmids. p_toxT_ harbors the toxT ORF on low-copy-number plasmid pMMB66HE under the control of the IPTG-inducible tac promoter. Protein molecular size standards (lane M) are indicated on the left. w.t., wild type.
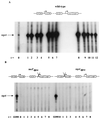
FIG. 5
RNA primer extension analyses of V. cholerae mutant strains Δ_tcpP_/pMMB66EH and Δ_tcpP_/p_tcpPH_ (A) and mutant strain Δ_tcpH_ (B). Samples were collected at 1-h intervals after back dilution of a culture grown overnight in LB medium at 30°C 1:100 into fresh LB medium. IPTG (1 mM) was used to induce transcription in strains carrying plasmids. A radiolabeled toxT primer was used. RNA collected from wild-type strain O395 after 2 to 3 h of growth was used as a positive control for all of the mutant strains. p_tcpPH_ harbors the tcpP and tcpH ORFs on low-copy-number plasmid pMMB66EH under the control of the IPTG-inducible tac promoter.
FIG. 6
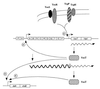
Model for control of toxT transcription and coordinate regulation of virulence in V. cholerae. ToxR, ToxS, TcpP, and TcpH in the inner membrane activate toxT transcription from the toxT promoter. ToxT protein then activates transcription of the tcpA promoter, producing more ToxT from the readthrough transcript. ToxT also activates transcription of other virulence genes, including ctx, as shown here. The tcp gene cluster and the ctx operon are shown with each box representing a gene. Symbols: , promoter; ∧∧∧→, transcript of the relevant genes; ∥○ stem-loop structure which is a putative RNA-processing site for the readthrough transcript initiating from the tcpA promoter.
Similar articles
- Vibrio pathogenicity island and cholera toxin genetic element-associated virulence genes and their expression in non-O1 non-O139 strains of Vibrio cholerae.
Sarkar A, Nandy RK, Nair GB, Ghose AC. Sarkar A, et al. Infect Immun. 2002 Aug;70(8):4735-42. doi: 10.1128/IAI.70.8.4735-4742.2002. Infect Immun. 2002. PMID: 12117994 Free PMC article. - Organization of tcp, acf, and toxT genes within a ToxT-dependent operon.
Brown RC, Taylor RK. Brown RC, et al. Mol Microbiol. 1995 May;16(3):425-39. doi: 10.1111/j.1365-2958.1995.tb02408.x. Mol Microbiol. 1995. PMID: 7565104 - Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation.
Yu RR, DiRita VJ. Yu RR, et al. Mol Microbiol. 2002 Jan;43(1):119-34. doi: 10.1046/j.1365-2958.2002.02721.x. Mol Microbiol. 2002. PMID: 11849541 - Regulation of virulence in Vibrio cholerae.
Klose KE. Klose KE. Int J Med Microbiol. 2001 May;291(2):81-8. doi: 10.1078/1438-4221-00104. Int J Med Microbiol. 2001. PMID: 11437342 Review. - Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae.
DiRita VJ. DiRita VJ. Mol Microbiol. 1992 Feb;6(4):451-8. doi: 10.1111/j.1365-2958.1992.tb01489.x. Mol Microbiol. 1992. PMID: 1560773 Review.
Cited by
- Host cell contact induces expression of virulence factors and VieA, a cyclic di-GMP phosphodiesterase, in Vibrio cholerae.
Dey AK, Bhagat A, Chowdhury R. Dey AK, et al. J Bacteriol. 2013 May;195(9):2004-10. doi: 10.1128/JB.02127-12. Epub 2013 Feb 22. J Bacteriol. 2013. PMID: 23435982 Free PMC article. - Activation of prophage eib genes for immunoglobulin-binding proteins by genes from the IbrAB genetic island of Escherichia coli ECOR-9.
Sandt CH, Hopper JE, Hill CW. Sandt CH, et al. J Bacteriol. 2002 Jul;184(13):3640-8. doi: 10.1128/JB.184.13.3640-3648.2002. J Bacteriol. 2002. PMID: 12057959 Free PMC article. - Differential activation of the tcpPH promoter by AphB determines biotype specificity of virulence gene expression in Vibrio cholerae.
Kovacikova G, Skorupski K. Kovacikova G, et al. J Bacteriol. 2000 Jun;182(11):3228-38. doi: 10.1128/JB.182.11.3228-3238.2000. J Bacteriol. 2000. PMID: 10809704 Free PMC article. - Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade.
Nye MB, Pfau JD, Skorupski K, Taylor RK. Nye MB, et al. J Bacteriol. 2000 Aug;182(15):4295-303. doi: 10.1128/JB.182.15.4295-4303.2000. J Bacteriol. 2000. PMID: 10894740 Free PMC article. - Regulated Proteolysis in Vibrio cholerae Allowing Rapid Adaptation to Stress Conditions.
Pennetzdorfer N, Lembke M, Pressler K, Matson JS, Reidl J, Schild S. Pennetzdorfer N, et al. Front Cell Infect Microbiol. 2019 Jun 21;9:214. doi: 10.3389/fcimb.2019.00214. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31293982 Free PMC article. Review.
References
- Betley M J, Miller V L, Mekalanos J J. Genetics of bacterial enterotoxins. Annu Rev Microbiol. 1986;40:577–605. - PubMed
- Brown R C, Taylor R K. Organization of the tcp, acf, and toxT genes within a ToxT-dependent operon. Mol Microbiol. 1995;16:425–439. - PubMed
- Carroll P A, Tashima K T, Rogers M B, DiRita V J, Calderwood S B. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol Microbiol. 1997;25:1099–1111. - PubMed
- Champion G A, Neely M N, Brennan M A, DiRita V J. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol Microbiol. 1997;23:323–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR 00200/RR/NCRR NIH HHS/United States
- K01 RR000200/RR/NCRR NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- T32 GM007544/GM/NIGMS NIH HHS/United States
- T32 GM 07544/GM/NIGMS NIH HHS/United States
- AI 31645/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources