Stable alphavirus packaging cell lines for Sindbis virus and Semliki Forest virus-derived vectors - PubMed (original) (raw)
. 1999 Apr 13;96(8):4598-603.
doi: 10.1073/pnas.96.8.4598.
B A Belli, D A Driver, I Frolov, S Sherrill, M J Hariharan, K Townsend, S Perri, S J Mento, D J Jolly, S M Chang, S Schlesinger, T W Dubensky Jr
Affiliations
- PMID: 10200308
- PMCID: PMC16378
- DOI: 10.1073/pnas.96.8.4598
Stable alphavirus packaging cell lines for Sindbis virus and Semliki Forest virus-derived vectors
J M Polo et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Alphavirus vectors are being developed for possible human vaccine and gene therapy applications. We have sought to advance this field by devising DNA-based vectors and approaches for the production of recombinant vector particles. In this work, we generated a panel of alphavirus vector packaging cell lines (PCLs). These cell lines were stably transformed with expression cassettes that constitutively produced RNA transcripts encoding the Sindbis virus structural proteins under the regulation of their native subgenomic RNA promoter. As such, translation of the structural proteins was highly inducible and was detected only after synthesis of an authentic subgenomic mRNA by the vector-encoded replicase proteins. Efficient production of biologically active vector particles occurred after introduction of Sindbis virus vectors into the PCLs. In one configuration, the capsid and envelope glycoproteins were separated into distinct cassettes, resulting in vector packaging levels of 10(7) infectious units/ml, but reducing the generation of contaminating replication-competent virus below the limit of detection. Vector particle seed stocks could be amplified after low multiplicity of infection of PCLs, again without generating replication-competent virus, suggesting utility for production of large-scale vector preparations. Furthermore, both Sindbis virus-based and Semliki Forest virus-based vectors could be packaged with similar efficiency, indicating the possibility of developing a single PCL for use with multiple alphavirus-derived vectors.
Figures
Figure 1
Schematic illustration of Sindbis virus structural protein expression cassettes used to generate PCL. Sindbis virus-derived sequences shown in white include the 5′ end (wild type or defective interfering-derived) and 3′ end cis replication elements, subgenomic RNA promoter (junction region, JR), structural protein genes (C, pE2, and E1), poly(A) tract and remaining nonstructural gene sequences deleted of nucleotides 422 (_Bsp_EI site) to 7335 (_Bam_HI site). Shaded regions indicate other elements, including: RNA polymerase II promoter (PRO), hepatitis delta virus antigenomic ribozyme (δ rbz), bovine growth hormone transcription termination signal (TT/pA), SV40 small t antigen intron with splice donor (SD), and splice acceptor (s.a.) sites, neomycin or hygromycin phosphotransferase gene (neor or hygror, respectively), and SV40-driven neomycin phosphotransferase gene (SVneo). The fusion protein generated with neor and remaining nonstructural protein 1 (nsP1) amino acids is expanded to show more detail.
Figure 2
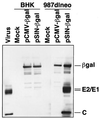
Western blot analysis of Sindbis virus structural proteins expressed in PCLs. Cell lysates were obtained from parental BHK-21 cells and 987dlneo PCLs 48 hr after transfection with a SIN-βgal DNA vector (induced), a CMV-βgal vector (uninduced), or mock transfection. Equivalent cell lysates were separated by SDS/PAGE and probed with a mixture of Sindbis virion and β-gal-specific rabbit polyclonal antisera. Positive control lysates were obtained from wild-type Sindbis virus-infected BHK-21 cells.
Figure 3
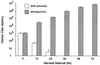
Vector particle amplification by a PCL. Parental BHK-21 cells and split structural protein gene PCL clone 24 were infected in triplicate with an RCV-free stock of SIN-βgal vector particles at a multiplicity of infection of 0.2. After a 1-hr infection period, the inoculum was removed, and cell monolayers were washed and replenished with fresh media. At the indicated intervals, culture supernatants were harvested for vector titer determination and replaced with fresh media. The titer of SIN-βgal vector particles produced during each period was quantitated by infecting naive BHK-21 cells and staining with X-gal (5-bromo-4-chloro-3-indolyl β-
d
-galactoside), as described in Materials and Methods.
Figure 4
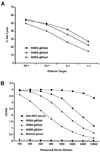
Induction of HSVgB-specific CTL (A) and antibody (B) responses in mice immunized with SIN-HSVgB vector particles. BALB/c mice were immunized once i.m. with the indicated doses of a SIN-HSVgB vector preparation free of RCV. Serum was obtained at 17 days postimmunization and tested for antibody by ELISA. Splenocytes from individual mice were obtained at week 4, stimulated in vitro with retroviral vector-transduced BC10ME (H-2d) cells expressing HSVgB, and tested separately for specific CTL lysis by using BC10ME target cells expressing either HSVgB or β-gal. Percent specific target cell lysis was calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100, and graphs show specific lysis after subtraction of β-gal target cell background. Values shown within each dosage group represent the mean of four mice.
Similar articles
- Selection and characterization of packaging cell lines for XJ-160 virus.
Zhu WY, Liang GD. Zhu WY, et al. Intervirology. 2009;52(2):100-6. doi: 10.1159/000215947. Epub 2009 May 7. Intervirology. 2009. PMID: 19420962 - Properties and use of novel replication-competent vectors based on Semliki Forest virus.
Rausalu K, Iofik A, Ulper L, Karo-Astover L, Lulla V, Merits A. Rausalu K, et al. Virol J. 2009 Mar 24;6:33. doi: 10.1186/1743-422X-6-33. Virol J. 2009. PMID: 19317912 Free PMC article. - Therapeutic and prophylactic applications of alphavirus vectors.
Atkins GJ, Fleeton MN, Sheahan BJ. Atkins GJ, et al. Expert Rev Mol Med. 2008 Nov 11;10:e33. doi: 10.1017/S1462399408000859. Expert Rev Mol Med. 2008. PMID: 19000329 Review. - Generation of recombinant alphaviral vectors.
Lundstrom K. Lundstrom K. Cold Spring Harb Protoc. 2012 Jul 1;2012(7):825-31. doi: 10.1101/pdb.prot070151. Cold Spring Harb Protoc. 2012. PMID: 22753600 - Alphavirus vectors for gene therapy applications.
Lundstrom K. Lundstrom K. Curr Gene Ther. 2001 May;1(1):19-29. doi: 10.2174/1566523013349039. Curr Gene Ther. 2001. PMID: 12109136 Review.
Cited by
- Modulation of disease, T cell responses, and measles virus clearance in monkeys vaccinated with H-encoding alphavirus replicon particles.
Pan CH, Valsamakis A, Colella T, Nair N, Adams RJ, Polack FP, Greer CE, Perri S, Polo JM, Griffin DE. Pan CH, et al. Proc Natl Acad Sci U S A. 2005 Aug 16;102(33):11581-8. doi: 10.1073/pnas.0504592102. Epub 2005 Jul 21. Proc Natl Acad Sci U S A. 2005. PMID: 16037211 Free PMC article. - Comparison of the immune responses induced by chimeric alphavirus-vectored and formalin-inactivated alum-precipitated measles vaccines in mice.
Bergen MJ, Pan CH, Greer CE, Legg HS, Polo JM, Griffin DE. Bergen MJ, et al. PLoS One. 2010 Apr 22;5(4):e10297. doi: 10.1371/journal.pone.0010297. PLoS One. 2010. PMID: 20421972 Free PMC article. - Reverse genetics of measles virus and resulting multivalent recombinant vaccines: applications of recombinant measles viruses.
Billeter MA, Naim HY, Udem SA. Billeter MA, et al. Curr Top Microbiol Immunol. 2009;329:129-62. doi: 10.1007/978-3-540-70523-9_7. Curr Top Microbiol Immunol. 2009. PMID: 19198565 Free PMC article. Review. - An alphavirus replicon particle chimera derived from venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector.
Perri S, Greer CE, Thudium K, Doe B, Legg H, Liu H, Romero RE, Tang Z, Bin Q, Dubensky TW Jr, Vajdy M, Otten GR, Polo JM. Perri S, et al. J Virol. 2003 Oct;77(19):10394-403. doi: 10.1128/jvi.77.19.10394-10403.2003. J Virol. 2003. PMID: 12970424 Free PMC article. - Vaccines for Venezuelan equine encephalitis.
Paessler S, Weaver SC. Paessler S, et al. Vaccine. 2009 Nov 5;27 Suppl 4:D80-5. doi: 10.1016/j.vaccine.2009.07.095. Vaccine. 2009. PMID: 19837294 Free PMC article. Review.
References
- Huang H V. Curr Opin Biotechnol. 1996;7:531–535. - PubMed
- Xiong C, Levis R, Shen P, Schlesinger S, Rice C M, Huang H V. Science. 1989;243:1188–1191. - PubMed
- Liljeström P, Garoff H. Biotechnology. 1991;9:1356–1361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials