Cumulative effect of phosphorylation of pRB on regulation of E2F activity - PubMed (original) (raw)
Cumulative effect of phosphorylation of pRB on regulation of E2F activity
V D Brown et al. Mol Cell Biol. 1999 May.
Abstract
The product of the retinoblastoma susceptibility gene, pRB, is a nuclear phosphoprotein that controls cell growth by binding to and suppressing the activities of transcription factors such as the E2F family. Transactivation activity is inhibited when E2F is bound to hypophosphorylated pRB and released when pRB is phosphorylated by cyclin-dependent kinases (CDKs). To determine which of 16 potential CDK phosphorylation sites regulated the pRB-E2F interaction, mutant pRB proteins produced by site-directed mutagenesis were tested for the ability to suppress E2F-mediated transcription in a reporter chloramphenicol acetyltransferase assay. Surprisingly, no one CDK site regulated the interaction of pRB with E2F when E2F was bound to DNA. Instead, disruption of transcriptional repression resulted from accumulation of phosphate groups on the RB molecule.
Figures
FIG. 1
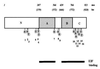
Diagram of murine pRB. Numbers above the schematic designate the amino acids. The A and B regions required for binding the SV40 large T antigen are shown. Positions of the potential phosphorylation sites (S [serine] and T [threonine]), numbered sequentially, in the protein are shown. The bars at the bottom represent the regions required for pRB to bind E2F on DNA.
FIG. 2
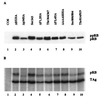
(A) Expression of pRB mutant constructs. Ten micrograms of RB plasmid was transfected into COS-7 cells. Quantities of cell lysates corrected for transfection efficiency by using cotransfected pCMVβgal were immunoprecipitated with 10 μl of anti-HA antibody 12CA5 followed by Western blotting with anti-HA antibody. The underphosphorylated (pRB) and hyperphosphorylated (ppRB) forms of pRB are indicated. (B) Orthophosphate labeling of pRB mutants. At 36 h after transfection of pRB mutants, COS-7 cells were incubated in 1.2 ml of phosphate-deficient medium, containing 800 μCi of 32PO4 per ml at 37°C for 4.5 h. Phosphorylated pRB was immunoprecipitated with anti-HA antibody 12CA5, loaded on an SDS–7.5% polyacrylamide gel, and visualized by autoradiography. Coprecipitated T antigen (TAg) is indicated.
FIG. 3
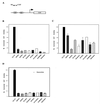
Coimmunoprecipitation of E2F1 with pRB mutants. Ten micrograms of RB plasmid was transfected with 0.5 μg of pCMV-E2F1 in C33A cells. Quantities of cell lysates corrected for transfection efficiency by using cotransfected pCMVβgal were immunoprecipitated with 5 μl of anti-pRB antibody (Pharmingen 14001A). pRB and coprecipitating proteins were separated on an SDS–7.5% polyacrylamide gel. The top half of the nitrocellulose was Western blotted with anti-HA antibody 12CA5, and the bottom half was Western blotted with anti-E2F1 (Santa Cruz C-20). The asterisk represents immunoglobulin G. Lane 1, untransfected C33A lysate (10% of the quantity used in immunoprecipitation); lane 2, immunoprecipitation of untransfected C33A lysate; lane 3, C33A lysate transfected with pCMV-E2F1 alone (10% of the quantity used in immunoprecipitation); lanes 4 to 17, immunoprecipitation of lysates transfected as indicated.
FIG. 4
Repression of the E2 (−80/−70)-CAT reporter construct. (A) Schematic of the E2(−80/−70)CAT reporter construct. Three micrograms of E2(−80/−70)-CAT was transfected into C33A cells with 3 μg of each pRB construct (B) as well as 5 μg each of cyclin E and cdk2 constructs (C). One microgram of CMVβgal was included in the transfection to normalize CAT activity for transfection efficiency. SVLuc was used as filler DNA to prevent promoter competition. The activity obtained with the reporter construct in the absence of RB plasmid (B) and in the presence of cyclin E and cdk2 (C) is arbitrarily set to 100%. The ability of each pRB mutant to suppress transcription was determined by reduction in CAT activity. Error bars represent standard errors of two to four experiments. (D) C33A cells were transfected as for panel B, with roscovitine added at a final concentration of 700 nM 18 h following transfection. The activity obtained with the reporter construct in the presence of roscovitine is arbitrarily set to 100%.
FIG. 5
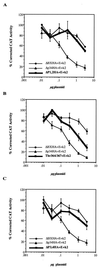
Cyclin E-cdk2 regulation of pRB mutants on E2F-mediated transcription. Various amounts of RB plasmid were cotransfected with 3 μg of E2(−80/−70)-CAT construct into C33A cells in the presence of 5 μg each of cyclin E (E) and cdk2 (k2) constructs. Promoter activity was determined as for Fig. 3. (A) ΔP1,2HA; (B) Thr364/367; (C) ΔP3,4HA. Error bars represent the standard errors of two to four experiments.
FIG. 6
Regulation of E2F-mediated transcription by ΔK6 (A) and ΔK11 (B). Various amounts of RB plasmid were cotransfected with 3 μg of E2(−80/−70)-CAT construct into C33A cells in the presence of 5 μg each of cyclin E (E) and cdk2 (k2) constructs. Promoter activity was determined as for Fig. 4.
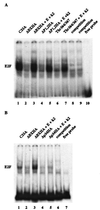
FIG. 7
Gel mobility shift of pRB mutants. Three micrograms of RB plasmid was transfected into C33A cells in the absence or presence of 5 μg of cyclin E (E) and cdk2 (k2) plasmids as indicated above the lanes. Nuclear extracts were prepared as described in Materials and Methods. Quantities of extracts corrected for transfection efficiency by using cotransfected pCMVβgal were added to a 32P-labeled oligonucleotide containing an E2F site; complexes were resolved on a 4% native polyacrylamide gel. Competition of the E2F binding activity was performed with a 50-fold excess of unlabeled oligonucleotide.
References
- Antelman D, Perry S, Hollingsworth R, Gregory R J, Driscoll B, Fung Y K, Bookstein R. Engineered mutants of pRB with improved growth suppression potential. Oncogene. 1997;15:2855–2866. - PubMed
- Bagchi S, Weinmann R, Raychaudhuri P. The retinoblastoma protein copurifies with E2F-1, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991;65:1063–1070. - PubMed
- Beijersbergen R L, Kerkhoven R M, Zhu L, Carlee L, Voorhoeve P M, Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–2690. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases