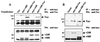hSiah2 is a new Vav binding protein which inhibits Vav-mediated signaling pathways - PubMed (original) (raw)
hSiah2 is a new Vav binding protein which inhibits Vav-mediated signaling pathways
A Germani et al. Mol Cell Biol. 1999 May.
Abstract
The hematopoietic proto-oncogene vav has been characterized as a Rac1-GDP/GTP exchanger protein which regulates cytoskeletal reorganization as well as signaling pathways leading to the activation of stress-activated protein kinases (SAPK/JNKs). Furthermore, vav overexpression enhances basal and T-cell receptor (TCR)-mediated stimulation of the nuclear factor of activated T cells (NFAT). We report here the interaction between Vav and hSiah2, a mammalian homolog of Drosophila Seven in absentia (Sina) that has been implicated in R7 photoreceptor cell formation during Drosophila eye development via the proteasome degradation pathway. Vav and hSiah2 interact in vitro and in vivo and colocalize in the cytoplasm of hematopoietic cells. The Src homology domain of Vav and the C-terminal region of hSiah2 are required for this interaction. We provide evidence for a negative regulation by hSiah2 of Vav-induced basal and TCR-mediated NFAT-dependent transcription. Overexpression of hSiah2 also inhibits the onco-Vav-induced JNK activation. Although the Vav-interacting domain is located in the C-terminal portion of hSiah2, the N-terminal region of hSiah2 is necessary for the inhibitory role that seems to be independent of the proteasome degradation.
Figures
FIG. 1
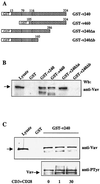
Vav interacts with hSiah2 in the yeast two-hybrid system. (A) Schematic representation of hSiah2 and the clones obtained from the two-hybrid screening. (B) Protein interaction in the two-hybrid system. The L40 reporter strain was cotransformed with 1 μg of the indicated pLex- and pGAD-derived plasmids, and interactions were detected as β-galactosidase activity.
FIG. 2
In vitro binding between hSiah2 and Vav. (A) Schematic representation of GST-hSiah2 fusion proteins. (B) Binding of Vav to GST-hSiah2 fusion proteins. Total-cell lysates from 107 Jurkat T cells were incubated at 4°C for 2 h with 1 μg of the fusion proteins or GST alone. (C) (Top) Lysates from unstimulated (0 min) and CD3-plus-CD28-stimulated (1 min and 30 min) Jurkat T cells (107) were incubated with 1 μg of GST-v240. The resulting complexes were resolved by SDS-PAGE, and the Western blot (Wb) was developed with anti-Vav MAb. The left-hand lane contains total lysate from 2 × 105 cells. (Bottom) Western blot analysis with antiphosphotyrosine antibody (anti-PTyr) of the total-cell extracts used in the top panel.
FIG. 3
hSiah2 interacts with Vav in mammalian cells. COS-7 cells (A) or T-Ag Jurkat cells (B), transiently transfected with 4 and 20 μg, respectively, of either pKES-Vav (Vav), myc-tagged pCAN-v240 (myc-v240), or pCAN-v460 (myc-v460) alone or with a combination of the vectors (Vav+myc-v240; Vav+myc-v460), were lysed in NP-40 buffer and immunoprecipitated with anti-myc MAb and protein G-Sepharose beads. Immunoprecipitates (IP) were detected with anti-Vav MAb (top). Expression of transfected hSiah2 (clones v240 and v460) was verified by reprobing the nitrocellulose membrane with anti-myc MAb (bottom). Wb, Western blot.
FIG. 4
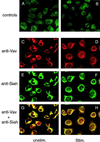
Immunolocalization of Vav and hSiah2 by confocal immunofluorescence microscopy. RBL cells were labeled with preimmune Siah antiserum (A), Siah antiserum depleted of the immunizing peptide (B), anti-Vav MAb (C and D), and anti-hSiah2 rabbit polyclonal antibody (E and F) as described in Materials and Methods. Colocalization of red fluorescence from Vav and green fluorescence from hSiah2 produced a yellow signal, indicating an overlap in the distribution of the two proteins (G and H). In panels D, F, and H, cells were stimulated (Stim.) by FcɛRI cross-linking. Panels A and B were obtained with a much higher transmission rate in order for the signal to be detectable.
FIG. 5
Vav-mediated NFAT activation is inhibited by hSiah2. (A) T-Ag Jurkat cells (107) were transfected with NFAT and pSVβ-galactosidase reporter plasmids (5 and 1 μg, respectively) and 20 μg of either an empty vector (vector), pEF-Vav (Vav), pCAN-v240 (v240), pCAN-v460 (v460), or a combination of the vectors as indicated. A total of 106 cells were either left unstimulated or stimulated after 24 h with anti-CD3 plus anti-CD28 for 8 h. Luciferase activity was measured and corrected for β-galactosidase activity, and the results were expressed as average fold induction relative to unstimulated cells transfected with the empty vector. The data are representative of four independent experiments. The basal activity and the maximum NFAT responses were approximately 600 and 2 × 105 AU, respectively. (B) T-Ag Jurkat cell lysates from panel A were analyzed by immunoblotting for expression of Vav and hSiah2 (v240 and v460). Wb, Western blot.
FIG. 6
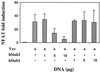
Overexpression of hSiah2 inhibits Vav-induced NFAT activity in a dose-dependent manner. T-Ag Jurkat cells (107) were transfected with NFAT and pSVβ-galactosidase reporter plasmids (5 and 1 μg, respectively), 10 μg of pEF-Vav (Vav), and increasing concentrations of pBK-CMV hSiah2 or pBK-CMV hSiah1 as indicated. Luciferase activity was determined and normalized to β-galactosidase activity to correct for transfection efficiency.
FIG. 7
Inhibitory effect of hSiah2 on onco-Vav-mediated JNK activation. COS-7 (A) or T-Ag Jurkat (C) cells were transfected with 1 μg (A) or 5 μg (C) of pcDNA3-HA-JNK together with 3 μg (A) or 15 μg (C) of expression vectors containing cDNA for the indicated plasmids. The total amount of transfected DNA was kept constant by using empty pcDNA3 vector. COS-7 cells treated with 1 μg of anisomycin per ml for 20 min were used as a control. The kinase reaction was performed in anti-HA immunoprecipitates from the corresponding cellular lysates with purified GST–c-Jun as a substrate (A and C, top panels). The levels of HA-JNK protein were confirmed by Western blot (Wb) analysis with anti-HA antibody (bottom panels). Values in the histogram represent the means and the standard errors of four independent experiments. COS-7 (B) and T-Ag Jurkat (D) cell lysates from panels A and C were analyzed by immunoblotting for expression of Vav and myc-epitope-tagged hSiah2 (v240 and v460). The additional bands in these blots could be due to degradative events caused by onco-Vav overexpression.
FIG. 8
hSiah2 does not decrease the half-life of Vav. COS-7 cells were cotransfected with 4 μg of myc-tagged pEF-Vav (myc-Vav) and pCAN-v240 (myc-v240) or pCAN-v460 (myc-v460). At 48 h after transfection, the cells were pulse-labeled for 1 h with [35S]methionine, chased with cold methionine for the indicated times, and then lysed as described in Materials and Methods. Vav and hSiah2 proteins were immunoprecipitated (IP) with anti-myc antibody and analyzed by SDS-PAGE and autoradiography.
Similar articles
- Regulatory and signaling properties of the Vav family.
Bustelo XR. Bustelo XR. Mol Cell Biol. 2000 Mar;20(5):1461-77. doi: 10.1128/MCB.20.5.1461-1477.2000. Mol Cell Biol. 2000. PMID: 10669724 Free PMC article. Review. No abstract available. - A Nck-Pak1 signaling module is required for T-cell receptor-mediated activation of NFAT, but not of JNK.
Yablonski D, Kane LP, Qian D, Weiss A. Yablonski D, et al. EMBO J. 1998 Oct 1;17(19):5647-57. doi: 10.1093/emboj/17.19.5647. EMBO J. 1998. PMID: 9755165 Free PMC article. - Vav-Rac1-mediated activation of the c-Jun N-terminal kinase/c-Jun/AP-1 pathway plays a major role in stimulation of the distal NFAT site in the interleukin-2 gene promoter.
Kaminuma O, Deckert M, Elly C, Liu YC, Altman A. Kaminuma O, et al. Mol Cell Biol. 2001 May;21(9):3126-36. doi: 10.1128/MCB.21.9.3126-3136.2001. Mol Cell Biol. 2001. PMID: 11287617 Free PMC article. - Vav-induced activation of the human IFN-gamma gene promoter is mediated by upregulation of AP-1 activity.
Kaminuma O, Elly C, Tanaka Y, Mori A, Liu YC, Altman A, Miyatake S. Kaminuma O, et al. FEBS Lett. 2002 Mar 13;514(2-3):153-8. doi: 10.1016/s0014-5793(02)02316-5. FEBS Lett. 2002. PMID: 11943142 - [The role of vav protein in TCR-mediated signaling with MHC/peptide complexes leading to positive or negative selection of thymocytes].
Matuszyk J, Kałas W, Kozicki R, Strzadała L. Matuszyk J, et al. Postepy Hig Med Dosw. 1999;53(4):531-43. Postepy Hig Med Dosw. 1999. PMID: 10544657 Review. Polish.
Cited by
- The RING finger protein Siah-1 regulates the level of the transcriptional coactivator OBF-1.
Tiedt R, Bartholdy BA, Matthias G, Newell JW, Matthias P. Tiedt R, et al. EMBO J. 2001 Aug 1;20(15):4143-52. doi: 10.1093/emboj/20.15.4143. EMBO J. 2001. PMID: 11483517 Free PMC article. - Regulatory and signaling properties of the Vav family.
Bustelo XR. Bustelo XR. Mol Cell Biol. 2000 Mar;20(5):1461-77. doi: 10.1128/MCB.20.5.1461-1477.2000. Mol Cell Biol. 2000. PMID: 10669724 Free PMC article. Review. No abstract available. - Distinct expression patterns of the E3 ligase SIAH-1 and its partner Kid/KIF22 in normal tissues and in the breast tumoral processes.
Bruzzoni-Giovanelli H, Fernandez P, Veiga L, Podgorniak MP, Powell DJ, Candeias MM, Mourah S, Calvo F, Marín M. Bruzzoni-Giovanelli H, et al. J Exp Clin Cancer Res. 2010 Feb 9;29(1):10. doi: 10.1186/1756-9966-29-10. J Exp Clin Cancer Res. 2010. PMID: 20144232 Free PMC article. - S-nitrosylation of B23/nucleophosmin by GAPDH protects cells from the SIAH1-GAPDH death cascade.
Lee SB, Kim CK, Lee KH, Ahn JY. Lee SB, et al. J Cell Biol. 2012 Oct 1;199(1):65-76. doi: 10.1083/jcb.201205015. J Cell Biol. 2012. PMID: 23027902 Free PMC article. - The ubiquitin ligase component Siah1a is required for completion of meiosis I in male mice.
Dickins RA, Frew IJ, House CM, O'Bryan MK, Holloway AJ, Haviv I, Traficante N, de Kretser DM, Bowtell DD. Dickins RA, et al. Mol Cell Biol. 2002 Apr;22(7):2294-303. doi: 10.1128/MCB.22.7.2294-2303.2002. Mol Cell Biol. 2002. PMID: 11884614 Free PMC article.
References
- Adams M D, Dubnick M, Kerlavage A R, Moreno R, Kelley J M, Utterback T R, Nagle J W, Fields C, Venter J C. Sequence identification of 2,375 human brain genes. Nature. 1992;355:632–634. - PubMed
- Altman, M. Personal communication.
- Amson R B, Nemani M, Roperch J P, Israeli D, Bougueleret L, Le Gall I, Medhioub M, Linares-Cruz G, Lethrosne F, Pasturaud P, Piouffre L, Prieur S, Susini L, Alvaro V, Millasseau P, Guidicelli C, Bui H, Massart C, Cazes L, Dufour F, Bruzzoni-Giovanelli H, Owadi H, Hennion C, Charpak G, Telerman A, et al. Isolation of 10 differentially expressed cDNAs in p53-induced apoptosis: activation of the vertebrate homologue of the Drosophila seven in absentia gene. Proc Natl Acad Sci USA. 1996;93:3953–3957. - PMC - PubMed
- Angel P, Allegretto E A, Okino S T, Hattori K, Boyle W J, Hunter T, Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988;332:166–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous