Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3 - PubMed (original) (raw)
Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3
H Ellinger-Ziegelbauer et al. Mol Cell Biol. 1999 May.
Abstract
Signal-induced proliferation, differentiation, or stress responses of cells depend on mitogen-activated protein kinase (MAPK) cascades, the core modules of which consist of members of three successively acting kinase families (MAPK kinase kinase [MAP3K], MAPK kinase, and MAPK). It is demonstrated here that the MEKK3 kinase inhibits cell proliferation, a biologic response not commonly associated with members of the MAP3K family of kinases. A conditionally activated form of MEKK3 stably expressed in fibroblasts arrests these cells in early G1. MEKK3 critically blocks mitogen-driven expression of cyclin D1, a cyclin which is essential for progression of fibroblasts through G1. The MEKK3-induced block of cyclin D1 expression and of cell cycle progression may be mediated via p38 MAPK, a downstream effector of MEKK3. The MEKK3-mediated block of proliferation also reverses Ras-induced cellular transformation, suggesting possible tumor-suppressing functions for this kinase. Together, these results suggest an involvement of the MEKK3 kinase in negative regulation of cell cycle progression, and they provide the first insights into biologic activities of this kinase.
Figures
FIG. 1
Activation of MEKK3 induces morphological changes and formation of cell processes. NIH 3T3 [MEKK3-ER] fibroblasts were seeded onto poly-
l
-lysine-coated coverslips and incubated in complete medium in the absence (A and B) or presence (C and D) of 1 μM E2. After 4 days, the cells were fixed and either photographed under phase-contrast optics (A and C) or stained with antitubulin antibodies by indirect immunofluorescence (B and D). Magnifications of corresponding top and bottom panels are identical. The number of cells in the presence of E2 (activated MEKK3-ER) is much lower than that observed in the absence of E2 (A and B). Activated MEKK3-ER induces cells to increase in size and to send out long processes. These processes appear to be stabilized by microtubule bundles (D).
FIG. 2
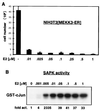
Activation of MEKK3 inhibits proliferation. (A) MEKK3-ER prevents cell division. NIH 3T3 [MEKK3-ER] cells were seeded in triplicate at 2 × 104 per well of a six-well plate in complete medium in the absence or presence of increasing concentrations of E2, as indicated. After 4 days, the attached cells were harvested by trypsinization, pooled with the detached cells, and counted in the presence of trypan blue to distinguish between live and dead cells. The percentage of dead cells was marginal, even with the highest concentration of E2. The total number of cells counted is indicated on the y axis (±mean standard deviation). As discussed in Results, no significant cell death could be measured. (B) Activation of SAPK by MEKK3-ER in the presence of increasing concentrations of E2. NIH 3T3 [MEKK3-ER] cells were transiently transfected with HA-tagged SAPK, serum starved overnight, and stimulated for 15 min with the indicated concentrations of E2. The activity of SAPK was measured in an immunocomplex kinase assay with GST–c-Jun as the substrate. After SDS-PAGE, phosphorylated GST–c-Jun was quantified with a Phosphorimager; data are expressed as fold activation relative to the signal obtained in the absence of E2. Western blot analysis confirmed equal expression of HA-SAPK in the different extracts (data not shown). Activation of SAPK and growth inhibition appear to occur at roughly the same low doses of E2.
FIG. 3
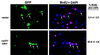
Activation of MEKK3 inhibits DNA synthesis. NIH 3T3 [MEKK3-ER] cells were starved in the absence of serum for 3 days with or without E2 and then stimulated with 10% FCS in the presence of 10 μM BrdU (with or without E2). (A) Cells were fixed and analyzed by indirect immunofluorescence for BrdU incorporation, shown in red. Nuclei were visualized with DAPI (blue). (B) Cells were processed for FACS analysis to quantitatively measure BrdU incorporation (% BrdU) and DNA content, the latter to determine the percentage of cells in different phases of the cell cycle. Similar results were obtained in two independent experiments. As demonstrated by both indirect immunofluorescence and FACS analysis, the number of cells actively synthesizing DNA is greatly reduced in the presence of active MEKK3.
FIG. 4
Activation of MEKK3 inhibits various molecular parameters of cell cycle progression, as determined by analysis of inhibition of cyclin expression and CAK activities. (A and D) NIH 3T3 [MEKK3-ER] cells were kept without serum for 4 days in the presence or absence of E2 and then stimulated with 10% FCS for 12 or 24 h in the presence or absence of E2, as indicated. For the Western blot shown in panel B, cells were continuously kept in medium containing 10% FCS in the presence or absence of E2. cycD1, cyclin D1. (C) Two different clones of PC12 cells stably expressing MEKK3-ER (#6 and #17) were starved in the absence of FCS for 1 day with or without E2 and then released into medium containing 10% FCS for 2 days (with or without E2), as indicated. (A to C) Detergent lysates (100 μg) were electrophoretically separated on denaturing polyacrylamide gels and immunoblotted with antibodies against the proteins indicated on the left. CD-LBD designates the MEKK3-ER fusion protein, and cdk2* designates the T160 phosphorylated form of cdk2. (D) An aliquot of 100 μg (for cdk4 kinase assay) or 50 μg (all others) of extract was immunoprecipitated (IP) with the indicated antibodies and then assayed for associated kinase activity in an immunocomplex kinase assay with histone H1 or GST-pRb as the substrate.
FIG. 5
Importance of cyclin D1 downregulation in MEKK3-mediated inhibition of cell cycle progression. NIH 3T3 [MEKK3-ER] cells were transfected with GFP and either vector control or cyclin D1 (cycD1) and cdk4 expression vectors. One day after transfection, cells were kept without serum for 30 h in the presence of E2 and then incubated in complete medium with E2 and 10 μM BrdU for 15 h to label cells actively synthesizing DNA. Incorporated BrdU was detected by indirect immunofluorescence (right panel, red nuclei), and nuclei were identified with the general DAPI stain (right panel, blue nuclei). Transfected cells are identified by the green fluorescence of GFP (left panels). On the right the percentage of BrdU-positive cells in the transfected population is shown as the average (±standard deviation) after counting about 800 cells each from either vector- or cyclin D1-plus-cdk4-transfected cells from three different experiments. The arrows mark three examples of transfected cells for each of the two sets of transfections; most of the vector-only transfected cells (top left panel; GFP) were BrdU negative (top right panel), while cells transfected with cyclin D1 and cdk4 (bottom left panel) were mostly BrdU positive (bottom right panel). In this transient transfection experiment, more of the E2-activated NIH 3T3 [MEKK3-ER] cells were BrdU positive than what was observed for the E2-activated NIH 3T3 [MEKK3-ER] cells in the experiment shown in Fig. 3, because the transiently transfected cells could not be starved long enough to make all of them quiescent prior to serum stimulation.
FIG. 6
Transient activation of MAPKs by MEKK3. NIH 3T3 [MEKK3-ER] cells were serum starved for 2 days and then stimulated with E2 or serum for the indicated times. (A) Endogenous ERK activity (top panel) was measured in an immunocomplex kinase assay after immunoprecipitation with a mixture of antibodies recognizing ERK1 and ERK2, with MBP as the substrate. For comparison, the cells were also stimulated with serum. Kinase activity was determined with a PhosphorImager by measuring the amount of radioactivity that was incorporated into MBP; the data are presented as fold activation relative to the activity seen in unstimulated cells. The amount of extract used after serum stimulation was only 20% (30 μg) of that used after E2 treatment (150 μg). Thus, the maximal ERK activation achieved by MEKK3-ER is only about 7% of that obtained with serum stimulation. Western blot (WB) analysis with an anti-ERK2 antibody (bottom panel) which cross-reacts with ERK1 shows that equal amounts of ERK1 and ERK2 are present in the extracts. (B) MKP-1 expression is induced by MEKK3-ER. The Western blot shown in panel A was stripped and restained with an anti-MKP-1 antibody. (C) The activation state of endogenous p38 and endogenous SAPK was determined by Western blotting with antibodies recognizing only the active forms of the kinases (active p38 and active SAPK). An identical Western blot stained with anti-p38 and anti-SAPK revealed that the total amounts of these kinases do not vary among the samples.
FIG. 7
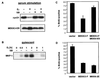
MEKK3 inhibits cyclin D1 expression and G1 progression via p38. (A) p38 activity is necessary for inhibition of cyclin D1 induction by MEKK3-ER. NIH 3T3 [MEKK3-ER] cells were kept without serum for 4 days, preincubated for 30 min with or without 10 μM SB203580 (SB), incubated in the presence of E2 for another 2 h, and then stimulated with 10% FCS in the presence or absence of SB203580 and/or E2 for 12 h. Equal amounts of protein (100 μg) were separated by SDS-PAGE and then sequentially immunoblotted with an anti-cyclin D1 antibody (cycD1), followed by an anti-estrogen receptor antibody to detect the stably expressed fusion protein (MEKK3-ER). (B) MEKK3-induced expression of MKP-1 is largely dependent on p38 activity. NIH 3T3 [MEKK3-ER] cells were rendered quiescent by being cultured for 2 days without serum and then were stimulated with E2 for various times, as indicated, without serum. Before addition of E2, some cells were preincubated with 10 μM SB203580 (SB) as well. MKP-1 expression was detected by Western analysis (100 μg/lane) with anti-Pac1 antibody, which cross-reacts with MKP-1. (C) Inhibition of G1/S progression by wild-type MEKK3 but relief of inhibition in the presence of MKK6[KN]. NIH 3T3 cells were transfected with GFP expression plasmid alone or together with 0.1 μg of an expression plasmid expressing full-length MEKK3 (MEKK3-F), in the absence or presence of 0.3 μg of an expression vector expressing MKK6[KN], as indicated. After transfection, the cells were incubated overnight, serum starved for 30 h, and then stimulated with 10% FCS in the presence of BrdU. The bars represent percentages of BrdU-positive cells in the transfected, GFP-positive population (±mean standard deviation of duplicate sets). More than 100 cells were counted for each sample, and similar results were obtained in two independent experiments. (D) Wild-type MEKK3 and a constitutively active form of MKK6 inhibit G1/S progression to similar extents. NIH 3T3 cells were transfected with 0.1 μg of GFP expression plasmid and 0.2 μg of an expression vector encoding MEKK3-F or MKK6[2E], a constitutively active form of MKK6. The cells were treated and evaluated as for panel C. In all experiments, total amounts of transfected DNA were kept constant with the addition of empty vectors as needed.
FIG. 8
MEKK3 reverses cell-transforming activities of oncogenic Ras. NIH 3T3 [MEKK3-ER] cells were infected with a recombinant retrovirus expressing constitutively active RasQ61L and used as a pool of infected cells for all experiments. (A) Uninfected (left panels) or RasQ61L-infected (right panels) NIH 3T3 [MEKK3-ER] cells were seeded at low density in the absence of E2 (top panels) or when confluent in the presence of E2 (bottom panels). After 4 days when cells minus E2 had reached confluency as well, photographs were taken with Hoffmann optics, which gives a three-dimensional image of the cell surface. Ras-transformed cells show the characteristic rounded and spindle-shaped morphology (top right panel) which is largely reverted to a flat morphology in the presence of E2 (bottom panel right). (B) NIH 3T3 [MEKK3-ER] cells transformed with RasQ61L were tested for anchorage-independent growth in the presence (bottom panel) or absence (top panel) of E2, corresponding to active or inactive MEKK3-ER, respectively. Two weeks after seeding photographs were taken and the percentage of colonies consisting of at least five cells was determined in a total of 20 different fields from four different dishes and in two independent experiments, each with or without E2. (C) Untransformed (MEKK3) or RasQ61L-transformed (MEKK3 + ras) NIH 3T3 [MEKK3-ER] cells were starved in the absence of serum for 3.5 days with or without E2 and then either stimulated or not stimulated with 10% FCS for 15 h, with or without the continued presence E2, as indicated. Detergent lysates (100 μg) were separated by SDS-PAGE and immunoblotted with antibodies against cyclin D1 (cycD1), cyclin A, endogenous MEKK3, or the MEKK3-ER fusion protein, as indicated on the left. Ras-transformed cells express the G1-phase cyclin D1 even in the absence of serum, which is suppressed in the presence of E2 and active MEKK3-ER. Serum stimulation further increases cyclin D1 expression, but only in the absence of E2.
FIG. 8
MEKK3 reverses cell-transforming activities of oncogenic Ras. NIH 3T3 [MEKK3-ER] cells were infected with a recombinant retrovirus expressing constitutively active RasQ61L and used as a pool of infected cells for all experiments. (A) Uninfected (left panels) or RasQ61L-infected (right panels) NIH 3T3 [MEKK3-ER] cells were seeded at low density in the absence of E2 (top panels) or when confluent in the presence of E2 (bottom panels). After 4 days when cells minus E2 had reached confluency as well, photographs were taken with Hoffmann optics, which gives a three-dimensional image of the cell surface. Ras-transformed cells show the characteristic rounded and spindle-shaped morphology (top right panel) which is largely reverted to a flat morphology in the presence of E2 (bottom panel right). (B) NIH 3T3 [MEKK3-ER] cells transformed with RasQ61L were tested for anchorage-independent growth in the presence (bottom panel) or absence (top panel) of E2, corresponding to active or inactive MEKK3-ER, respectively. Two weeks after seeding photographs were taken and the percentage of colonies consisting of at least five cells was determined in a total of 20 different fields from four different dishes and in two independent experiments, each with or without E2. (C) Untransformed (MEKK3) or RasQ61L-transformed (MEKK3 + ras) NIH 3T3 [MEKK3-ER] cells were starved in the absence of serum for 3.5 days with or without E2 and then either stimulated or not stimulated with 10% FCS for 15 h, with or without the continued presence E2, as indicated. Detergent lysates (100 μg) were separated by SDS-PAGE and immunoblotted with antibodies against cyclin D1 (cycD1), cyclin A, endogenous MEKK3, or the MEKK3-ER fusion protein, as indicated on the left. Ras-transformed cells express the G1-phase cyclin D1 even in the absence of serum, which is suppressed in the presence of E2 and active MEKK3-ER. Serum stimulation further increases cyclin D1 expression, but only in the absence of E2.
Similar articles
- Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway.
Lavoie JN, L'Allemain G, Brunet A, Müller R, Pouysségur J. Lavoie JN, et al. J Biol Chem. 1996 Aug 23;271(34):20608-16. doi: 10.1074/jbc.271.34.20608. J Biol Chem. 1996. PMID: 8702807 - Delta MEKK3:ER* activation induces a p38 alpha/beta 2-dependent cell cycle arrest at the G2 checkpoint.
Garner AP, Weston CR, Todd DE, Balmanno K, Cook SJ. Garner AP, et al. Oncogene. 2002 Nov 21;21(53):8089-104. doi: 10.1038/sj.onc.1206000. Oncogene. 2002. PMID: 12444545 - Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence.
Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, Sun P. Wang W, et al. Mol Cell Biol. 2002 May;22(10):3389-403. doi: 10.1128/MCB.22.10.3389-3403.2002. Mol Cell Biol. 2002. PMID: 11971971 Free PMC article. - TGF-beta-activating kinase-1 inhibits cell cycle and expression of cyclin D1 and A in LLC-PK1 cells.
Terada Y, Nakashima O, Inoshita S, Kuwahara M, Sasaki S, Marumo F. Terada Y, et al. Kidney Int. 1999 Oct;56(4):1378-90. doi: 10.1046/j.1523-1755.1999.00665.x. Kidney Int. 1999. PMID: 10504490
Cited by
- Stress-stimulated mitogen-activated protein kinases control the stability and activity of the Cdt1 DNA replication licensing factor.
Chandrasekaran S, Tan TX, Hall JR, Cook JG. Chandrasekaran S, et al. Mol Cell Biol. 2011 Nov;31(22):4405-16. doi: 10.1128/MCB.06163-11. Epub 2011 Sep 19. Mol Cell Biol. 2011. PMID: 21930785 Free PMC article. - EZH2 regulates cofilin activity and colon cancer cell migration by targeting ITGA2 gene.
Ferraro A, Boni T, Pintzas A. Ferraro A, et al. PLoS One. 2014 Dec 30;9(12):e115276. doi: 10.1371/journal.pone.0115276. eCollection 2014. PLoS One. 2014. PMID: 25549357 Free PMC article. - Chemoprevention activity of dipyridamole in the MMTV-PyMT transgenic mouse model of breast cancer.
Wang C, Schwab LP, Fan M, Seagroves TN, Buolamwini JK. Wang C, et al. Cancer Prev Res (Phila). 2013 May;6(5):437-47. doi: 10.1158/1940-6207.CAPR-12-0345. Epub 2013 Feb 27. Cancer Prev Res (Phila). 2013. PMID: 23447563 Free PMC article. - Homeostatic interactions between MEKK3 and TAK1 involved in NF-kappaB signaling.
Di Y, Li S, Wang L, Zhang Y, Dorf ME. Di Y, et al. Cell Signal. 2008 Apr;20(4):705-13. doi: 10.1016/j.cellsig.2007.12.007. Epub 2008 Jan 18. Cell Signal. 2008. PMID: 18206350 Free PMC article. - p38(MAPK): stress responses from molecular mechanisms to therapeutics.
Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. Coulthard LR, et al. Trends Mol Med. 2009 Aug;15(8):369-79. doi: 10.1016/j.molmed.2009.06.005. Epub 2009 Aug 6. Trends Mol Med. 2009. PMID: 19665431 Free PMC article. Review.
References
- Albanese C, Johnson J, Watanabe G, Eklund N, Dzuy V, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
- Billon N, van Grunsven L A, Rudkin B B. The cdk inhibitor p21WAF1/CIP1 is induced through a p300-dependent mechanism during NGF-induced neuronal differentiation of PC12 cells. Oncogene. 1996;13:2047–2054. - PubMed
- Blank J L, Gerwins P, Elliott E M, Sather S, Johnson G L. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. J Biol Chem. 1996;271:5361–5368. - PubMed
- Brondello J M, McKenzie F R, Sun H, Tonks N K, Pouyssegur J. Constitutive MAP kinase phosphatase (MKP-1) expression blocks G1 specific gene transcription and S-phase entry in fibroblasts. Oncogene. 1995;10:1895–1904. - PubMed
- Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous