Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses - PubMed (original) (raw)
Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses
T Dohi et al. J Exp Med. 1999.
Abstract
To investigate the potential involvement of T helper (Th)2-type responses in murine models of intestinal inflammation, we used trinitrobenzene sulfonic acid (TNBS)-hapten to induce inflammatory bowel disease in situations where Th1-type responses with interferon (IFN)-gamma synthesis are either diminished or do not occur. Intracolonic administration of TNBS to either normal (IFN-gamma+/+) or Th1-deficient IFN-gamma knockout (IFN-gamma-/-) BALB/c mice resulted in significant colitis. In IFN-gamma-/- mice, crypt inflammation was more severe than in IFN-gamma+/+ mice and was accompanied by hypertrophy of colonic patches with a lymphoepithelium containing M cells and distinct B and T cell zones resembling Peyer's patches. Hapten-specific, colonic patch T cells from both mouse groups exhibited a Th2 phenotype with interleukin (IL)-4 and IL-5 production. TNBS colitis in normal mice treated with anti-IL-4 antibodies or in IL-4(-/-) mice was less severe than in either IFN-gamma+/+ or IFN-gamma-/- mice. Our findings now show that the Th2-type responses in TNBS colitis are associated with colonic patch enlargement and inflammation of the mucosal layer and may represent a model for ulcerative colitis.
Figures
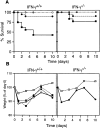
Figure 1
The course of TNBS colitis in normal and IFN-γ–deficient mice. (A) Survival rate of mice given TNBS enema. IFN-γ+/+ (left) or IFN-γ−/− (right) mice were given 50 (▪), 36 (•), or 25 μg TNBS/g weight (○) intracolonically on days 0 and 7. Each group contained 16–18 mice. The survival rate after administration of 50 μg/g weight was significantly higher in IFN-γ−/− than in IFN-γ+/+ mice. (B) Wasting disease in mice given TNBS enema. Left, weight loss of IFN-γ+/+ mice after administration of ethanol only (□), 36 μg/g weight TNBS–ethanol (○), TNBS–ethanol together with intraperitoneal administration of rat anti– IFN-γ antibody XMG1.2 (▪), or rat IgG as a control (•) on days 0 and 7. Right, weight loss of IFN-γ−/− mice given TNBS–ethanol (•) or ethanol only (□). The data shown represent average values, and each group contained 5–10 mice. The weights of mice treated with ethanol only were significantly higher than in other groups on days 1, 3, 5, and 10 (left) and on days 1, 3, 7, and 10 (right).
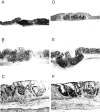
Figure 2
Histologic features of TNBS-induced colitis. (A–C) IFN-γ+/+ mice; (D–F) IFN-γ−/− mice. A and D show normal colon with colonic patches, and B, C, E, and F illustrate the colon after a TNBS enema. Original magnification was 10 in A, B, D, and E and 40 in C and F.
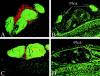
Figure 3
Immunohistochemistry of colonic lymphoid follicles of mice with TNBS colitis. The distribution of T (red) and B (green) cells in a colonic patch from an IFN-γ+/+ (A) or IFN-γ−/− (C) mouse. Serial sections were stained with PNA in B (IFN-γ+/+) and D (IFN-γ−/−). Original magnification was 100.
Figure 4
M cells detected in a colonic lymphoid follicle of an IFN-γ−/− mouse after TNBS enema. Irregular microvilli with attached bacteria and an underlying macrophage are shown. Bar, 5 μm.
Figure 5
TNBS-specific proliferative responses in IFN-γ+/+ or IFN-γ−/− mice with TNBS colitis. (A) Proliferative responses of GALT and SLN cells. Cells were prepared from small intestinal Peyer's patches (white bar), cecal patches (dotted bar), colonic lamina propria lymphocytes (gray bar), colonic patches (black bar), and SLN (hatched bar). The stimulation index was determined as cpm of wells with antigen (TNBS)/cpm of wells without TNBS. The level of [3H]thymidine incorporation for control wells without TNBS was <600 cpm. Values are shown as the average ± 1 SD and are representative of three separate experiments using pooled cells from 3–5 mice in each group. (B) Expression of cytokine-specific mRNA in TNBS-stimulated CD4+ T cells. TNBS-stimulated total cell suspensions, which were prepared from SLN or colonic patches (CP) from IFN-γ+/+ (lane 1) or IFN-γ−/− (lane 2) mice with TNBS colitis, were cultured for 24 h. CD4+ T cells were purified by sorting and subjected to cytokine-specific RT-PCR.
Figure 6
Expression of cytokine-specific mRNA from freshly isolated lamina propria lymphocytes. Total colonic lamina propria lymphocytes or CD4+ T cells purified by flow cytometry were prepared on days 0 (before TNBS enema), 1, and 10 and subjected to cytokine-specific RT-PCR. 50 ng of total RNA was used for each reaction. Representative results from three individual experiments are shown.
Figure 7
Histological scores for Th1 and Th2 cytokine-deficient mice with TNBS colitis. (A) IFN-γ–deficient mice; (B) IL-4–deficient mice. Each group included 4–10 mice. Values are shown as the average ± 1 SD. The score of IFN-γ−/− mice was significantly higher than that of other groups in A (P < 0.02). IL-4−/− mice and mice treated with anti–IL-4 mAb showed significantly lower values than did normal BALB/c and IFN-γ−/− mice (P < 0.05). −, rectal administration of ethanol only.
Similar articles
- Elimination of colonic patches with lymphotoxin beta receptor-Ig prevents Th2 cell-type colitis.
Dohi T, Rennert PD, Fujihashi K, Kiyono H, Shirai Y, Kawamura YI, Browning JL, McGhee JR. Dohi T, et al. J Immunol. 2001 Sep 1;167(5):2781-90. doi: 10.4049/jimmunol.167.5.2781. J Immunol. 2001. PMID: 11509623 - Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance.
Neurath MF, Fuss I, Kelsall BL, Presky DH, Waegell W, Strober W. Neurath MF, et al. J Exp Med. 1996 Jun 1;183(6):2605-16. doi: 10.1084/jem.183.6.2605. J Exp Med. 1996. PMID: 8676081 Free PMC article. - Evidence for the critical role of interleukin-12 but not interferon-gamma in the pathogenesis of experimental colitis in mice.
Tozawa K, Hanai H, Sugimoto K, Baba S, Sugimura H, Aoshi T, Uchijima M, Nagata T, Koide Y. Tozawa K, et al. J Gastroenterol Hepatol. 2003 May;18(5):578-87. doi: 10.1046/j.1440-1746.2003.03024.x. J Gastroenterol Hepatol. 2003. PMID: 12702051 - Immune responses of IL-4, IL-5, IL-6 deficient mice.
Kopf M, Le Gros G, Coyle AJ, Kosco-Vilbois M, Brombacher F. Kopf M, et al. Immunol Rev. 1995 Dec;148:45-69. doi: 10.1111/j.1600-065x.1995.tb00093.x. Immunol Rev. 1995. PMID: 8825282 Review. No abstract available. - Targeting IL-13 as a Host-Directed Therapy Against Ulcerative Colitis.
Hoving JC. Hoving JC. Front Cell Infect Microbiol. 2018 Nov 6;8:395. doi: 10.3389/fcimb.2018.00395. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30460209 Free PMC article. Review.
Cited by
- Management of ulcerative colitis by dichloroacetate: Impact on NFATC1/NLRP3/IL1B signaling based on bioinformatics analysis combined with in vivo experimental verification.
Abdel-Razek EA, Mahmoud HM, Azouz AA. Abdel-Razek EA, et al. Inflammopharmacology. 2024 Feb;32(1):667-682. doi: 10.1007/s10787-023-01362-2. Epub 2023 Oct 30. Inflammopharmacology. 2024. PMID: 37902927 Free PMC article. - Human intestinal B cells in inflammatory diseases.
Spencer J, Bemark M. Spencer J, et al. Nat Rev Gastroenterol Hepatol. 2023 Apr;20(4):254-265. doi: 10.1038/s41575-023-00755-6. Epub 2023 Feb 27. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36849542 Review. - Monitoring Gut Epithelium Serotonin and Melatonin Overflow Provides Spatial Mapping of Inflammation.
Perez F, Kotecha N, Lavoie B, Mawe GM, Patel BA. Perez F, et al. Chembiochem. 2023 Jan 17;24(2):e202200334. doi: 10.1002/cbic.202200334. Epub 2022 Dec 2. Chembiochem. 2023. PMID: 36394122 Free PMC article. - Inflammatory Bowel Disease: A Review of Pre-Clinical Murine Models of Human Disease.
Katsandegwaza B, Horsnell W, Smith K. Katsandegwaza B, et al. Int J Mol Sci. 2022 Aug 19;23(16):9344. doi: 10.3390/ijms23169344. Int J Mol Sci. 2022. PMID: 36012618 Free PMC article. Review. - Lactobacillus plantarum strains attenuated DSS-induced colitis in mice by modulating the gut microbiota and immune response.
Khan I, Wei J, Li A, Liu Z, Yang P, Jing Y, Chen X, Zhao T, Bai Y, Zha L, Li C, Ullah N, Che T, Zhang C. Khan I, et al. Int Microbiol. 2022 Aug;25(3):587-603. doi: 10.1007/s10123-022-00243-y. Epub 2022 Apr 12. Int Microbiol. 2022. PMID: 35414032
References
- Braegger CP, MacDonald TT. Immune mechanisms in chronic inflammatory bowel disease. Ann Allergy. 1994;72:135–141. - PubMed
- Brandtzaeg P, Haraldsen G, Rugtveit J. Immunopathology of human inflammatory bowel disease. Springer Semin Immunopathol. 1997;18:555–589. - PubMed
- Emmrich J, Seyfarth M, Fleig WE, Emmrich F. Treatment of inflammatory bowel disease with anti-CD4 monoclonal antibody. Lancet. 1991;338:570–571. - PubMed
- Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+T cells. J Exp Med. 1993;178:237–244. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI 43197/AI/NIAID NIH HHS/United States
- DK 44240/DK/NIDDK NIH HHS/United States
- R01 DE012242/DE/NIDCR NIH HHS/United States
- R01 AI018958/AI/NIAID NIH HHS/United States
- R29 DE012242/DE/NIDCR NIH HHS/United States
- AI 18958/AI/NIAID NIH HHS/United States
- P01 DK044240/DK/NIDDK NIH HHS/United States
- R01 AI043197/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases