Lineage-specific requirement for signal transducer and activator of transcription (Stat)4 in interferon gamma production from CD4(+) versus CD8(+) T cells - PubMed (original) (raw)
Comparative Study
Lineage-specific requirement for signal transducer and activator of transcription (Stat)4 in interferon gamma production from CD4(+) versus CD8(+) T cells
L L Carter et al. J Exp Med. 1999.
Abstract
CD4(+) and CD8(+) T cells exhibit important differences in their major effector functions. CD8(+) T cells provide protection against pathogens through cytolytic activity, whereas CD4(+) T cells exert important regulatory activity through production of cytokines. However, both lineages can produce interferon (IFN)-gamma, which can contribute to protective immunity. Here we show that CD4(+) and CD8(+) T cells differ in their regulation of IFN-gamma production. Both lineages require signal transducer and activator of transcription (Stat)4 activation for IFN-gamma induced by interleukin (IL)-12/IL-18 signaling, but only CD4(+) T cells require Stat4 for IFN-gamma induction via the TCR pathway. In response to antigen, CD8(+) T cells can produce IFN-gamma independently of IL-12, whereas CD4(+) T cells require IL-12 and Stat4 activation. Thus, there is a lineage-specific requirement for Stat4 activation in antigen-induced IFN-gamma production based on differences in TCR signaling between CD4(+) and CD8(+) T cells.
Figures
Figure 1
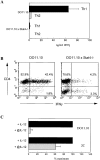
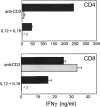
CD4+ T cells exhibit strict requirement for IL-12 and Stat4 in antigen-induced IFN-γ production. (A) Splenocytes from DO11.10 or DO11.10 × Stat4-deficient (Stat4−/−) were cultured with IL-2 and OVA, and either IL-12 (Th1) or IL-4 (Th2) as indicated for 7 d. Cells were restimulated with irradiated BALB/c splenocytes and OVA for 40 h and supernatants were assayed for IFN-γ. Data shown are the mean ± SD of four replicate determinations and is representative of three similar experiments. (B) Cells were treated as above except Brefeldin A was added for the final 4 h of a 19-h restimulation. Samples were stained with FITC-conjugated anti-CD4 and biotin-conjugated KJ1-26 followed by cychrome-streptavidin and PE-conjugated anti–IFN-γ as described in Materials and Methods. Analysis gates were set on live KJ1-26+ cells. Quadrants are based on isotype control staining. (C) Sorted DO11.10 CD4+ T cells were stimulated with OVA/APCs, IL-2, and either IL-12 (black bars) or anti–IL-12 (gray bars). Sorted CD8+ T cells from 2C mice were stimulated with APCs, and either IL-12 (black bars) or anti–IL-12 (gray bars). After 6 d, equal numbers of DO11.10 and 2C T cells were reactivated in the absence of cytokines for 40 h and IFN-γ was measured. Data are the mean ± SD of IFN-γ production represented as a percentage of the IL-12–treated condition (% maximum) from four independent experiments.
Figure 2
CD8+ T cells exhibit Stat4-independent IFN-γ production. CD4+ (black bars) and CD8+ (gray bars) T cells were sort-purified from pooled lymph nodes and spleens of wild-type or Stat4-deficient (Stat4 −/−) mice and stimulated with IL-2, IL-12, and irradiated allogeneic splenocytes (H-2b) (left and middle) or plate-bound anti-CD3 and anti-CD28 (right). After 6 d, cells were harvested and reactivated at 4 × 105/ml with irradiated allogeneic APCs (left) or plate-bound anti-CD3 (middle and right), and IFN-γ was measured after 40 h. Data in left and middle panels are the mean ± SD pooled from six independent experiments. Data in right panel are the mean ± SD from one of two experiments.
Figure 3
CD4+ and CD8+ T cells have distinct requirements for Stat4 in TCR-induced IFN-γ production. CD4+ and CD8+ T cells were sorted from lymph nodes and spleens of wild-type or Stat4-deficient mice and stimulated in the presence of IL-2 and IL-12 with either irradiated allogeneic splenocytes (A) or plate-bound anti-CD3 and anti-CD28 (B). After 5 d, cells were restimulated with irradiated allogeneic APCs (A) or plate-bound anti-CD3 (B) for 17 h with Brefeldin A added for the final 5 h. Samples were stained with FITC-conjugated anti-CD4 or anti-CD8, fixed, permeabilized, and stained with PE-conjugated anti–IFN-γ. Syngeneic splenocytes were used as stimulators for a specificity control and no IFN-γ+ cells were detected (data not shown). Gates for analysis excluded dead cells and quadrants were set based on isotype control staining. The percentages displayed indicate the frequency of cells positive for IFN-γ in the CD4+ (top) or CD8+ (bottom) population, rather than the percentage of total cells. Data shown are representative of three independent experiments.
Figure 4
CD8+ T cells possess both Stat4-independent (TCR) and Stat4-dependent (IL-12/IL-18) pathways for IFN-γ induction. CD4+ (top) and CD8+ (bottom) T cells were sorted from lymph nodes and spleens of wild-type (black bars) or Stat4-deficient (gray bars) mice and stimulated with irradiated allogeneic splenocytes, IL-2, and IL-12. After 5 d, cells were harvested and equal cell numbers were restimulated by the addition of IL-12 and IL-18 or plate-bound anti-CD3 for 40 h, and IFN-γ was measured by ELISA. Data are the mean ± SD of four independent experiments.
Similar articles
- CD8 T cells in innate immune responses: using STAT4-dependent but antigen-independent pathways to gamma interferon during viral infection.
Suarez-Ramirez JE, Tarrio ML, Kim K, Demers DA, Biron CA. Suarez-Ramirez JE, et al. mBio. 2014 Oct 21;5(5):e01978-14. doi: 10.1128/mBio.01978-14. mBio. 2014. PMID: 25336459 Free PMC article. - T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells.
Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. Afkarian M, et al. Nat Immunol. 2002 Jun;3(6):549-57. doi: 10.1038/ni794. Epub 2002 May 13. Nat Immunol. 2002. PMID: 12006974 - An absolute requirement for STAT4 and a role for IFN-gamma as an amplifying factor in IL-12 induction of the functional IL-18 receptor complex.
Nakahira M, Tomura M, Iwasaki M, Ahn HJ, Bian Y, Hamaoka T, Ohta T, Kurimoto M, Fujiwara H. Nakahira M, et al. J Immunol. 2001 Aug 1;167(3):1306-12. doi: 10.4049/jimmunol.167.3.1306. J Immunol. 2001. PMID: 11466347 - Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4.
Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Watford WT, et al. Immunol Rev. 2004 Dec;202:139-56. doi: 10.1111/j.0105-2896.2004.00211.x. Immunol Rev. 2004. PMID: 15546391 Review. - IFN-gamma production by antigen-presenting cells: mechanisms emerge.
Frucht DM, Fukao T, Bogdan C, Schindler H, O'Shea JJ, Koyasu S. Frucht DM, et al. Trends Immunol. 2001 Oct;22(10):556-60. doi: 10.1016/s1471-4906(01)02005-1. Trends Immunol. 2001. PMID: 11574279 Review.
Cited by
- Signatures of immune dysfunction in HIV and HCV infection share features with chronic inflammation in aging and persist after viral reduction or elimination.
Lopez Angel CJ, Pham EA, Du H, Vallania F, Fram BJ, Perez K, Nguyen T, Rosenberg-Hasson Y, Ahmed A, Dekker CL, Grant PM, Khatri P, Maecker HT, Glenn JS, Davis MM, Furman D. Lopez Angel CJ, et al. Proc Natl Acad Sci U S A. 2021 Apr 6;118(14):e2022928118. doi: 10.1073/pnas.2022928118. Proc Natl Acad Sci U S A. 2021. PMID: 33811141 Free PMC article. - Dynamic Roles for IL-2-STAT5 Signaling in Effector and Regulatory CD4+ T Cell Populations.
Jones DM, Read KA, Oestreich KJ. Jones DM, et al. J Immunol. 2020 Oct 1;205(7):1721-1730. doi: 10.4049/jimmunol.2000612. J Immunol. 2020. PMID: 32958706 Free PMC article. Review. - Friend of GATA-1 represses GATA-3-dependent activity in CD4+ T cells.
Zhou M, Ouyang W, Gong Q, Katz SG, White JM, Orkin SH, Murphy KM. Zhou M, et al. J Exp Med. 2001 Nov 19;194(10):1461-71. doi: 10.1084/jem.194.10.1461. J Exp Med. 2001. PMID: 11714753 Free PMC article. - IFN-alpha is not sufficient to drive Th1 development due to lack of stable T-bet expression.
Ramos HJ, Davis AM, George TC, Farrar JD. Ramos HJ, et al. J Immunol. 2007 Sep 15;179(6):3792-803. doi: 10.4049/jimmunol.179.6.3792. J Immunol. 2007. PMID: 17785816 Free PMC article. - An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3.
Farrar JD, Ouyang W, Löhning M, Assenmacher M, Radbruch A, Kanagawa O, Murphy KM. Farrar JD, et al. J Exp Med. 2001 Mar 5;193(5):643-50. doi: 10.1084/jem.193.5.643. J Exp Med. 2001. PMID: 11238595 Free PMC article.
References
- Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. - PubMed
- Bancroft GJ, Schreiber RD, Bosma GC, Bosma MJ, Unanue ER. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987;139:1104–1107. - PubMed
- Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. Pathogen-induced Th1 phenotype development in CD4+ alpha beta-TCR transgenic T cells is macrophage dependent. Int Immunol. 1993;5:371–382. - PubMed
- Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous