A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory - PubMed (original) (raw)
A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory
S Blum et al. J Neurosci. 1999.
Abstract
Behavioral, biophysical, and pharmacological studies have implicated the hippocampus in the formation and storage of spatial memory. However, the molecular mechanisms underlying long-term spatial memory are poorly understood. In this study, we show that mitogen-activated protein kinase (MAPK, also called ERK) is activated in the dorsal, but not the ventral, hippocampus of rats after training in a spatial memory task, the Morris water maze. The activation was expressed as enhanced phosphorylation of MAPK in the pyramidal neurons of the CA1/CA2 subfield. In contrast, no increase in the percentage of phospho-MAPK-positive cells was detected in either the CA3 subfield or the dentate gyrus. The enhanced phosphorylation was observed only after multiple training trials but not after a single trial or after multiple trials in which the location of the target platform was randomly changed between each trial. Inhibition of the MAPK/ERK cascade in dorsal hippocampi did not impair acquisition, but blocked the formation of long-term spatial memory. In contrast, intrahippocampal infusion of SB203580, a specific inhibitor of the stress-activated MAPK (p38 MAPK), did not interfere with memory storage. These results demonstrate a MAPK-mediated cellular event in the CA1/CA2 subfields of the dorsal hippocampus that is critical for long-term spatial memory.
Figures
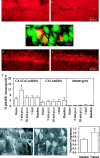
Fig. 1.
Behavioral training increases MAPK phosphorylation in the CA1/CA2 subfields of the dorsal hippocampus. Representative photomicrographs for phospho-MAPK immunoreactivity in the CA1/CA2 subfields of dorsal hippocampi (∼1.0 mm from the dorsal tip) from naive (a) and 5 min post-training (b) animals. Training to criterion increases the number of immunopositive cells for phospho-MAPK as compared with naive controls. c, Laser confocal image, indicating that the phospho-MAPK-positive cells (red) are also immunoreactive for a neuron-specific nuclear antigen (green). Areas with overlapping immunofluorescences appear yellow. Representative photomicrographs for MAPK immunoreactivity from a naive (d) and a 5 min post-training (_e)animal. f, Summary showing percentage of neurons staining positive for phosphorylated MAPK in the dorsal hippocampus from the control and experimental groups. The data are represented as the mean ± SEM (n = 5 for each group).g, Representative laser confocal images from a randomized platform and an animal trained to criterion and killed 5 min later, showing increased numbers of CA1/CA2 pyramidal neurons with nuclear staining for phospho-MAPK as a result of training. The_open and closed arrows indicate neurons with weak or strong phospho-MAPK nuclear immunoreactivity, respectively. The bar graph shows the nuclear to cytoplasmic ratio for phospho-MAPK immunofluroscence. *p ≤ 0.05;p.t.c., post-training to criterion.
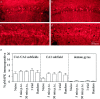
Fig. 2.
Behavioral training does not increase the percentage of phospho-MAPK-immunopositive cells in the ventral hippocampus. Representative photomicrographs of phospho-MAPK immunoreactivity in the CA1/CA2 subfields of ventral hippocampi (∼1.5 mm from the ventral tip) from naive (a) and 5 min post-training (b) animals. Representative photomicrographs for MAPK immunoreactivity in adjacent slices from naive (c) and 5 min-trained (d) animals. e, Summary figure showing the percentage of phospho-MAPK-immunopositive neurons in the CA1/CA2, CA3, and dentate gyrus subfields. The data are represented as the mean ± SEM (n = 5 for each group).p.t.c., Post-training to criterion.
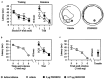
Fig. 3.
Infusion of PD098059 selectively decreases the phosphorylation of MAPK. a, Representative Western blots showing that infusion of PD098059 into the dorsal hippocampus decreases the phosphorylation, but not the total levels, of MAPK in the dorsal hippocampus when examined 30 min after infusion.b, Summary table showing that PD098059 inhibits MAPK but not SAPK phosphorylation in the dorsal hippocampus. Moreover, PD098059 infusion does not affect CaMK, PKC, or PKA activities (n = 4). *p ≤ 0.05 compared with vehicle controls. c, Representative photomicrographs showing decreased phospho-MAPK immunoreactivity of CA1/CA2 neurons in the dorsal hippocampus after PD098059 infusion as compared with the vehicle-infused contralateral side.
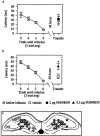
Fig. 4.
Inhibition of the MAPK cascade attenuates long-term retention in the Morris water maze task.a, Bilateral infusion of 2 μg/side PD098059 into the dorsal hippocampi 20 min before training does not interfere with acquisition but attenuates long-term retention (vehicle,n = 6; PD098059, n = 7). Note that the training sessions are plotted as blocks of four trials, whereas the retention trials are plotted individually. The inhibitor did not interfere with the subsequent reacquisition of the task, indicating that the drug does not interfere with visual acuity or motivational state. Representative traces from the first retention trial are shown. b, Post-training bilateral infusion of 2 μg/side PD098059 into dorsal hippocampi attenuates performance in the Morris water maze task when tested 48 hr later (vehicle,n = 12; PD098059, n = 13).c, Post-training infusion of 0.75 μg/side SB203580 (vehicle, n = 8; SB203580, n = 8) does not interfere with long-term memory.
Fig. 5.
a, Delayed (1 hr) infusion of 2 μg/side PD098059 does not interfere with long-term memory retention (vehicle, n = 6; PD098059, n = 6). b, Post-training bilateral infusion of 0.2 μg/side of PD098059 significantly attenuated performance 48 hr later as compared with vehicle (4% DMSO)-treated animals (vehicle,n = 8; PD098059, n = 8).c, Drawing of a coronal section of the hippocampus indicating sites (gray circles) of infusion for animals used in behavioral studies. Each _circle_represents a novel infusion site and may represent more than one animal. No sites were outside the boundary of the hippocampus.
Similar articles
- CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning.
Mizuno M, Yamada K, Maekawa N, Saito K, Seishima M, Nabeshima T. Mizuno M, et al. Behav Brain Res. 2002 Jul 18;133(2):135-41. doi: 10.1016/s0166-4328(01)00470-3. Behav Brain Res. 2002. PMID: 12110446 - Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus.
Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, Nishida E. Sugino T, et al. J Neurosci. 2000 Jun 15;20(12):4506-14. doi: 10.1523/JNEUROSCI.20-12-04506.2000. J Neurosci. 2000. PMID: 10844020 Free PMC article. - The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory.
Sweatt JD. Sweatt JD. J Neurochem. 2001 Jan;76(1):1-10. doi: 10.1046/j.1471-4159.2001.00054.x. J Neurochem. 2001. PMID: 11145972 Review. - Is the Ras-MAPK signalling pathway necessary for long-term memory formation?
Orban PC, Chapman PF, Brambilla R. Orban PC, et al. Trends Neurosci. 1999 Jan;22(1):38-44. doi: 10.1016/s0166-2236(98)01306-x. Trends Neurosci. 1999. PMID: 10088998 Review.
Cited by
- Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus.
Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Fortress AM, et al. Learn Mem. 2013 Feb 19;20(3):147-55. doi: 10.1101/lm.026732.112. Learn Mem. 2013. PMID: 23422279 Free PMC article. - Contributions of extracellular-signal regulated kinase 1/2 activity to the memory trace.
Ojea Ramos S, Feld M, Fustiñana MS. Ojea Ramos S, et al. Front Mol Neurosci. 2022 Oct 5;15:988790. doi: 10.3389/fnmol.2022.988790. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36277495 Free PMC article. Review. - Increases in cAMP, MAPK activity, and CREB phosphorylation during REM sleep: implications for REM sleep and memory consolidation.
Luo J, Phan TX, Yang Y, Garelick MG, Storm DR. Luo J, et al. J Neurosci. 2013 Apr 10;33(15):6460-8. doi: 10.1523/JNEUROSCI.5018-12.2013. J Neurosci. 2013. PMID: 23575844 Free PMC article. - Visual landmarks facilitate rodent spatial navigation in virtual reality environments.
Youngstrom IA, Strowbridge BW. Youngstrom IA, et al. Learn Mem. 2012 Feb 15;19(3):84-90. doi: 10.1101/lm.023523.111. Learn Mem. 2012. PMID: 22345484 Free PMC article. - Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation.
Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. Trifilieff P, et al. Learn Mem. 2006 May-Jun;13(3):349-58. doi: 10.1101/lm.80206. Epub 2006 May 16. Learn Mem. 2006. PMID: 16705140 Free PMC article.
References
- Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. - PubMed
- Alberini CM, Ghirardi M, Huang YY, Nguyen PV, Kandel ER. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann NY Acad Sci. 1995;758:261–286. - PubMed
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
- Amaral DG, Witter MP. The rat nervous system, Ed 2, pp 443–493. Academic; San Diego: 1995. Hippocampal formation.
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous