The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development - PubMed (original) (raw)
The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development
F A Pereira et al. Genes Dev. 1999.
Abstract
The embryonic expression of COUP-TFII, an orphan nuclear receptor, suggests that it may participate in mesenchymal-epithelial interactions required for organogenesis. Targeted deletion of the COUP-TFII gene results in embryonic lethality with defects in angiogenesis and heart development. COUP-TFII mutants are defective in remodeling the primitive capillary plexus into large and small microcapillaries. In the COUP-TFII mutant heart, the atria and sinus venosus fail to develop past the primitive tube stage. Reciprocal interactions between the endothelium and the mesenchyme in the vascular system and heart are essential for normal development of these systems. In fact, the expression of Angiopoietin-1, a proangiogenic soluble factor thought to mediate the mesenchymal-endothelial interactions during heart development and vascular remodeling, is down-regulated in COUP-TFII mutants. This down-regulation suggests that COUP-TFII may be required for bidirectional signaling between the endothelial and mesenchymal compartments essential for proper angiogenesis and heart development.
Figures
Figure 1
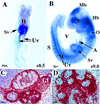
COUP-TFII expression. (A) Whole-mount in situ hybridization of an E8.5 embryo showing COUP-TFII expression in the expanding sinus venosus (Sv) and in the elongating umbilical veins (Uv) (ventral view, positive signal is bluish purple color). (H) Heart. (B) At E9, (E9.0), COUP-TFII is detected in the developing hindbrain (Hb), otocyst (O), the periocular mesenchyme and optic stalk (E), and expression in the anterior midbrain (Mb) is restricted to the neuroectoderm. Expression is also seen in the expanding common atrium (A), in the bilaterally symmetrical sinus venosus, and caudally in the developing umbilical veins. Expression in the somite (S), which was first detected at E8.5 (not shown), is now readily detected in all somites. (V) Ventricle. (C) COUP-TFII is expressed in the mesenchyme of the developing testis (T), genital tubercle (Gt), kidney (K), and cortex of the adrenal gland (Ag) at E13.5. Shown is a sagittal view of a double exposure with the positive signal in red. (D) COUP-TFII expression at E13.5 is restricted to the mesenchyme throughout salivary gland (Sg) development.
Figure 2
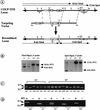
Targeted disruption of the COUP-TFII locus. (A) Targeting vector construction. (Shaded boxes I, II, and III) _COUP-TFII_-coding regions; (open box) 5′-untranslated region; (arrows in boxes) direction of transcription. (TK) Thymidine kinase gene; (_neo_r) neomycin-resistance gene. (R) _Eco_RI; (St) _Stu_I; (Sl) _Sal_I; (X) _Xba_I; (H) _Hin_dIII; (Sp) _Spe_I; (Xh) _Xho_I. (B) Southern blot analysis. (WT) Wild-type allele; (KO) recombined allele. Of the 680 colonies screened, 17 positive clones were obtained for a targeting efficiency of 2.5%. To ensure proper recombination had occurred at both the 5′ and 3′ ends of the _neo_r cassette, Southern analysis was performed with probes 5′ and 3′ of the targeting vector. The 5′ probe was a 0.8-kb _Eco_RI fragment with _Xba_I as the diagnostic enzyme as the _neo_r cassette (see A) introduced a new _Xba_I site to the locus. The expected 10-kb wild-type and 8-kb mutant fragments were generated (left). The 3′ probe was a 1-kb _Eco_RV–_Nsi_I fragment downstream of the 3′ homologous sequence and _Spe_I was the diagnostic enzyme that produced a 12-kb wild-type and a 6-kb mutant band (right). Reprobing the blots with the _neo_r gene revealed no other insertions in the genome (data not shown). Therefore, correct recombination was obtained at the COUP-TFII locus to inactivate the gene. (C) PCR genotyping showing wild-type (+/+), heterozygote (+/−), and mutant (−/−) embryos from the two independent (E5 and H7) COUP-TFII targeted lines. (D) RT–PCR analysis of transcripts from E9.5 embryos. There are no products in the absence of reverse transcriptase (RT) indicating that the bands are products of reverse transcription, and there are no wild-type COUP-TFII transcripts in the mutants (−/−). (KO) An amplified product from the 5′ end of the locus and the _neo_r gene.
Figure 3
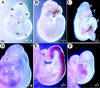
Severe hemorrhage in COUP-TFII mutants. Shown are whole-mount views of embryos isolated from COUP-TFII heterozygote matings at E9.5 (A_–_C) and E10.5 (D_–_F). Hemorrhaging occurs in the mutant forebrain (B and C) and throughout the brain vesicles (E) and heart (F). Note the edematous cysts that are often found in mutants (arrows in C). Mutants at E10.5 are growth retarded and are being resorbed as compared with wild-type (D). (Fb) Forebrain; (Mb) midbrain; (Hb) hindbrain; (O) otocyst; (Lb) limbbud; (S) somite; (H) heart.
Figure 4
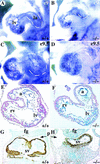
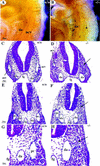
Defects in development of common atrium and sinus venosus. (A_–_D) Whole-mount PECAM immunostaining of hearts showing the left ventricle (lv) and atrium (la), which are demarcated by dashed lines. There is reduced staining in mutant ventricles at E9 (B), reflecting a reduced trabeculation in some mutant hearts. (C_–_D) At E9.5, there is a clear enlargement of the atrium in the wild type (C) whereas the mutant atrium is similar in size to an E9 embryo (D). (E_–_F) Sections through hearts showing an enlarged common atrial chamber (a) in the wild type (E) but there is only a primitive atrial tube in the mutant (F). (G_–_H) Abundant SMA immunostaining in the myocardium of the bilaterally symmetrical sinus venosus (Sv) that completely fills the pericardial–peritoneal cavity (p) in wild type (G). In the mutant, SMA staining shows the sinus venosus is a narrow tube leaving most of the pericardial–peritoneal cavity vacant (H). (fg) Foregut; (rv) right ventricle.
Figure 5
Malformation of cardinal veins (A,B) Whole-mount PECAM immunostaining showing a lack of the anterior cardinal vein (acv) in COUP-TFII mutants (asterisk in B) at E9.5. (C_–_H) Cross sections at the level of the otic vesicle (C,D) or at the midgut level (E_–_H). There is a lack of acv (arrow in D on left side of mutant embryo) and posterior cardinal vein (pc, arrow in F and H) or a reduced luminal diameter in the acv in D of mutants (right). (G,H) High magnifications of E and F. (da) Dorsal aorta; (fg) foregut.
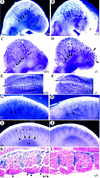
Figure 6
Angiogenesis defects. (A) A significantly remodeled vascular plexus in the wild type with formation of internal carotid artery (white arrow) and multiple branches of anterior cardinal vein (arrowheads) as revealed by whole-mount PECAM immunostaining. (B) There is only a primitive plexus in the mutant brain at E9 with vessels devoid of significant remodeling. Note the lack of vessels, irregular growth (asterisk), and edematous bubble in the frontonasal region. (C_–_L) At E9.5, there is continued vascular remodeling in the wild-type head (sagittal C; top E views), in the caudal hindbrain (G), and in the spine (I,K). In the mutant, there is poor remodeling, and some vessels have varicosities in the head (arrowheads in D), are distended (arrows in F,H), or are poorly branched (arrows in D and in the intersomitic region in J and L). (K,L) Wild-type and COUP-TFII mutants heterozygous for Tie1–LacZ and stained for β-gal (frontal sections) showing the lack of any vessels over the somites in mutants (L). (Lb) Limb bud; (D) dermamyotome; (IS) intersomitic vessels.
Figure 7
Down-regulation of Ang1 in COUP-TFII mutants. (A_–_B) Whole-mount in situ hybridization of Ang1 in wild type (A) and mutant (B) at E9.5. There is abundant Ang1 expression throughout the heart (H), frontonasal process (Fp), and periocular mesenchyme (E) in the wild type (A). In the COUP-TFII mutant, there is a dramatic reduction of Ang1 transcript in the frontonasal process and periocular mesenchyme and a significantly reduced expression in the ventricles and spine (B). (C) Semiquantitative mimic RT–PCR analysis of Ang1 transcripts in wild-type (+/+) and mutant (−/−) hearts as normalized to the level of L19 transcripts. Each lane represents amplified products of the endogenous gene and twofold serial dilution of the respective mimic plasmid. Note the 8- to 10-fold decrease of endogenous Ang1 transcripts in the mutant. (M) Size markers.
Similar articles
- The regulation of COUP-TFII gene expression by Ets-1 is enhanced by the steroid receptor co-activators.
Petit FG, Salas R, Tsai MJ, Tsai SY. Petit FG, et al. Mech Ageing Dev. 2004 Oct-Nov;125(10-11):719-32. doi: 10.1016/j.mad.2004.03.009. Mech Ageing Dev. 2004. PMID: 15541767 - The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development.
Lee CT, Li L, Takamoto N, Martin JF, Demayo FJ, Tsai MJ, Tsai SY. Lee CT, et al. Mol Cell Biol. 2004 Dec;24(24):10835-43. doi: 10.1128/MCB.24.24.10835-10843.2004. Mol Cell Biol. 2004. PMID: 15572686 Free PMC article. - Endocardial cushion morphogenesis and coronary vessel development require chicken ovalbumin upstream promoter-transcription factor II.
Lin FJ, You LR, Yu CT, Hsu WH, Tsai MJ, Tsai SY. Lin FJ, et al. Arterioscler Thromb Vasc Biol. 2012 Nov;32(11):e135-46. doi: 10.1161/ATVBAHA.112.300255. Epub 2012 Sep 6. Arterioscler Thromb Vasc Biol. 2012. PMID: 22962329 Free PMC article. - From apoptosis to angiogenesis: new insights into the roles of nuclear orphan receptors, chicken ovalbumin upstream promoter-transcription factors, during development.
Zhou C, Tsai SY, Tsai M. Zhou C, et al. Biochim Biophys Acta. 2000 Mar 27;1470(2):M63-8. doi: 10.1016/s0304-419x(00)00005-6. Biochim Biophys Acta. 2000. PMID: 10722928 Review. No abstract available.
Cited by
- Transcriptional Factors Mediating Retinoic Acid Signals in the Control of Energy Metabolism.
Zhang R, Wang Y, Li R, Chen G. Zhang R, et al. Int J Mol Sci. 2015 Jun 23;16(6):14210-44. doi: 10.3390/ijms160614210. Int J Mol Sci. 2015. PMID: 26110391 Free PMC article. Review. - COUP-TFII is a major regulator of cell cycle and Notch signaling pathways.
Chen X, Qin J, Cheng CM, Tsai MJ, Tsai SY. Chen X, et al. Mol Endocrinol. 2012 Aug;26(8):1268-77. doi: 10.1210/me.2011-1305. Epub 2012 Jun 25. Mol Endocrinol. 2012. PMID: 22734039 Free PMC article. - Transcriptional regulation of endothelial cell and vascular development.
Park C, Kim TM, Malik AB. Park C, et al. Circ Res. 2013 May 10;112(10):1380-400. doi: 10.1161/CIRCRESAHA.113.301078. Circ Res. 2013. PMID: 23661712 Free PMC article. Review. - Nuclear receptors in vascular biology.
Bishop-Bailey D. Bishop-Bailey D. Curr Atheroscler Rep. 2015 May;17(5):507. doi: 10.1007/s11883-015-0507-8. Curr Atheroscler Rep. 2015. PMID: 25772409 Review. - ARP-1 Regulates the Transcriptional Activity of the Aromatase Gene in the Mouse Brain.
Honda SI, Harada N. Honda SI, et al. Front Endocrinol (Lausanne). 2020 Jun 3;11:306. doi: 10.3389/fendo.2020.00306. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32582022 Free PMC article.
References
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): Alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. - PubMed
- Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Nüsslein-Volhard C. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. - PubMed
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. - PubMed
- Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous