The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors - PubMed (original) (raw)
The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors
L N Klapper et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The erbB-2/HER2 oncogene is overexpressed in a significant fraction of human carcinomas of the breast, ovary, and lung in a manner that correlates with poor prognosis. Although the encoded protein resembles several receptors for growth factors, no high affinity ligand of ErbB-2 has so far been fully characterized. However, several lines of evidence have raised the possibility that ErbB-2 can augment signal transduction initiated by binding of certain growth factors to their direct receptors. Here, we contrasted these two models of ErbB-2 function: First, examination of a large series of epidermal growth factor (EGF)-like ligands and neuregulins, including virus-encoded ligands as well as related motifs derived from the precursor of EGF, failed to detect interactions with ErbB-2 when this protein was singly expressed. Second, by using antibodies that block inter-ErbB interactions and cells devoid of surface ErbB-2, we learned that signaling by all ligands examined, except those derived from the precursor of EGF, was enhanced by the oncoprotein. These results imply that ErbB-2 evolved as a shared receptor subunit of all ErbB-specific growth factors. Thus, oncogenicity of ErbB-2 in human epithelia may not rely on the existence of a specific ligand but rather on its ability to act as a coreceptor for multiple stroma-derived growth factors.
Figures
Figure 1
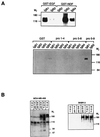
ErbB-2 activation depends on coexpression of other ErbB proteins. ErbB-2 phosphorylation was determined in cells expressing the receptor singly (A, D2) or in combination with ErbB-1 and ErbB-3 (B, SKOV3). The indicated ligands (100 ng/ml) or antibodies (20 μg/ml) were used to treat the cells for 5 min at 37°C. Receptor activation in whole cell lysates (A) or immunoprecipitates of ErbB-2 (B) was determined by an antibody directed against phosphorylated tyrosine.
Figure 2
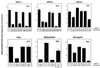
ErbB-2-dependency of growth stimulation by EGF-like ligands. 32D cells expressing ErbB-2 with either ErbB-1 (D12), ErbB-3 (D23), or ErbB-4 (D24) were tested for cell proliferation. Cells deprived of IL-3 were treated with the indicated ligands. Anti-ErbB-2 mAbs belonging to class I (L431), class II (L26, L96), class III (L140), and class IV (L87) or their respective Fab fragments (F26, F431) were added simultaneously. Alternatively, control antibodies were used, including an unrelated mAb (NR), mAbs capable of ligand displacement from ErbB-3 (C105) or ErbB-4 (C72, C36), or an antibody against ErbB-3 that is incapable of displacing NRGs (C379). The extent of cell proliferation was determined 24 h after the addition of stimulating factors by using the colorimetric 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. The results are presented as fold induction over control untreated cells and are the mean ± SD of eight determinations. Note that most mAbs (e.g., L26) have a weak agonist activity of their own.
Figure 3
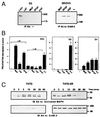
The effect of surface-expressed ErbB-2 on the kinetics of ligand-induced tyrosine phosphorylation and MAPK activation. ErbB ligands were used to stimulate T47D breast cancer cells and their derivative, T47D-5R, which lacks surface expression of ErbB-2. A comparable number of cells was stimulated at 37°C by the indicated ligands (at 100 ng/ml) for various time intervals. Receptor activation, in whole cell lysates, was detected by immunoblotting (IB) with an antibody directed against phosphorylated tyrosine (P-TYR). MAPK activation in the same preparations was determined by using an antibody against the active doubly phosphorylated form of Erk proteins (Activated MAPK). For control of equal gel loading, the upper part of membranes used to detect MAPK was used to determine the amount of ErbB-2. Note that the 5R cells exhibited up-regulation of the cell-retained ErbB-2.
Figure 4
Activation of ErbB receptors by EGF-like motifs of human proEGF. (A) GST fusion proteins containing EGF-like motifs 1–4, 5–8, or 5–9 of the EGF precursor were immobilized on glutathione-agarose beads. For control, GST fusion proteins containing EGF or NDF were used. The beads were incubated for 1 h at 4°C with conditioned media containing 1 μg of the indicated IgB protein. Protein complexes were immunoblotted with an anti-human Fc antiserum for detection of bound IgBs. (B) Monolayers of the indicated human breast cancer cell lines were incubated, for 10 min at 37°C, in the presence of 100 ng/ml GST fusion proteins or 5 ng/ml ligands (EGF or NDF). Receptor activation was detected by an antiphosphotyrosine antibody.
Figure 5
Viral peptides recruit ErbB-2. (A) Phosphorylation of ErbB-2 by viral peptides [vaccinnia virus growth factor (VGF), Myxoma virus growth factor (MGF), and SFGF] and antibodies (L87, L431) was examined as described in the legend to Fig. 1. (B) IL-3-deprived D23 cells were stimulated by viral peptides in the presence (+L26) or absence _(−_L26) of a class II mAb to the human ErbB-2 (Left). Cells singly expressing ErbB-2 (D2) or ErbB-1 (D1) served as negative and positive controls for ligand activity, respectively. Proliferation induction was determined by the 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay as described in the legend to Fig. 2. For control of endogenous proliferation signals, we incubated cells with IL-3. (C) The effect of ErbB-2 on downstream activation by SFGF was examined in cells that do (T47D) or do not (T47D-5R) express ErbB-2 on their surface. A time response of activation was detected in whole cell lysates by immunoblotting with an antibody against activated MAPK. The amount of ErbB-2 was verified by immunoblotting the upper part of the membrane with an antibody against the receptor.
Similar articles
- ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer.
Karunagaran D, Tzahar E, Beerli RR, Chen X, Graus-Porta D, Ratzkin BJ, Seger R, Hynes NE, Yarden Y. Karunagaran D, et al. EMBO J. 1996 Jan 15;15(2):254-64. EMBO J. 1996. PMID: 8617201 Free PMC article. - Insulin-like growth factor and epidermal growth factor independence in human mammary carcinoma cells with c-erbB-2 gene amplification and progressively elevated levels of tyrosine-phosphorylated p185erbB-2.
Ram TG, Dilts CA, Dziubinski ML, Pierce LJ, Ethier SP. Ram TG, et al. Mol Carcinog. 1996 Mar;15(3):227-38. doi: 10.1002/(SICI)1098-2744(199603)15:3<227::AID-MC8>3.0.CO;2-E. Mol Carcinog. 1996. PMID: 8597535 - Bivalence of EGF-like ligands drives the ErbB signaling network.
Tzahar E, Pinkas-Kramarski R, Moyer JD, Klapper LN, Alroy I, Levkowitz G, Shelly M, Henis S, Eisenstein M, Ratzkin BJ, Sela M, Andrews GC, Yarden Y. Tzahar E, et al. EMBO J. 1997 Aug 15;16(16):4938-50. doi: 10.1093/emboj/16.16.4938. EMBO J. 1997. PMID: 9305636 Free PMC article. - The achilles heel of ErbB-2/HER2: regulation by the Hsp90 chaperone machine and potential for pharmacological intervention.
Citri A, Kochupurakkal BS, Yarden Y. Citri A, et al. Cell Cycle. 2004 Jan;3(1):51-60. Cell Cycle. 2004. PMID: 14657666 Review. - The ErbB-2/HER2 oncogenic receptor of adenocarcinomas: from orphanhood to multiple stromal ligands.
Tzahar E, Yarden Y. Tzahar E, et al. Biochim Biophys Acta. 1998 Feb 20;1377(1):M25-37. doi: 10.1016/s0304-419x(97)00032-2. Biochim Biophys Acta. 1998. PMID: 9540810 Review.
Cited by
- Hyperinsulinemia promotes metastasis to the lung in a mouse model of Her2-mediated breast cancer.
Ferguson RD, Gallagher EJ, Cohen D, Tobin-Hess A, Alikhani N, Novosyadlyy R, Haddad N, Yakar S, LeRoith D. Ferguson RD, et al. Endocr Relat Cancer. 2013 May 21;20(3):391-401. doi: 10.1530/ERC-12-0333. Print 2013 Jun. Endocr Relat Cancer. 2013. PMID: 23572162 Free PMC article. - Mechanics of EGF receptor/ErbB2 kinase activation revealed by luciferase fragment complementation imaging.
Macdonald-Obermann JL, Piwnica-Worms D, Pike LJ. Macdonald-Obermann JL, et al. Proc Natl Acad Sci U S A. 2012 Jan 3;109(1):137-42. doi: 10.1073/pnas.1111316109. Epub 2011 Dec 21. Proc Natl Acad Sci U S A. 2012. PMID: 22190492 Free PMC article. - Monitoring activities of receptor tyrosine kinases using a universal adapter in genetically encoded split TEV assays.
Wintgens JP, Wichert SP, Popovic L, Rossner MJ, Wehr MC. Wintgens JP, et al. Cell Mol Life Sci. 2019 Mar;76(6):1185-1199. doi: 10.1007/s00018-018-03003-2. Epub 2019 Jan 8. Cell Mol Life Sci. 2019. PMID: 30623207 Free PMC article. - Tailored cancer immunotherapy using combinations of chemotherapy and a mixture of antibodies against EGF-receptor ligands.
Lindzen M, Lavi S, Leitner O, Yarden Y. Lindzen M, et al. Proc Natl Acad Sci U S A. 2010 Jul 13;107(28):12559-63. doi: 10.1073/pnas.1006218107. Epub 2010 Jun 28. Proc Natl Acad Sci U S A. 2010. PMID: 20616021 Free PMC article.
References
- van der Geer P, Hunter T, Lindberg R A. Annu Rev Cell Biol. 1994;10:251–337. - PubMed
- Burden S, Yarden Y. Neuron. 1997;18:847–855. - PubMed
- Salomon D S, Brandt R, Ciardiello F, Normanno N. Crit Rev Oncol Hematol. 1995;19:183–232. - PubMed
- Hynes N E, Stern D F. Biochim Biophys Acta. 1994;1198:165–184. - PubMed
- Klapper, L. N., Kirschbaum, M. H., Sela, M. & Yarden, Y. (1999) Adv. Cancer Res., in press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous