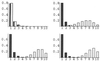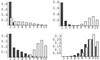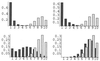Transmission bottlenecks as determinants of virulence in rapidly evolving pathogens - PubMed (original) (raw)
Transmission bottlenecks as determinants of virulence in rapidly evolving pathogens
C T Bergstrom et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Transmission bottlenecks occur in pathogen populations when only a few individual pathogens are transmitted from one infected host to another in the initiation of a new infection. Transmission bottlenecks can dramatically affect the evolution of virulence in rapidly evolving pathogens such as RNA viruses. Characterizing pathogen diversity with the quasispecies concept, we use analytical and simulation methods to demonstrate that severe bottlenecks are likely to drive down the virulence of a pathogen because of stochastic loss of the most virulent pathotypes, through a process analogous to Muller's ratchet. We investigate in this process the roles of host population size, duration of within-host viral replication, and transmission bottleneck size. We argue that the patterns of accumulation of deleterious mutation may explain differing levels of virulence in vertically and horizontally transmitted diseases.
Figures
Figure 1
The forces driving quasispecies evolution. Any given viral pathotype N is more likely to undergo deleterious mutation to pathotype N − 1 than it is to undergo advantageous mutation to pathotype N + 1. However, pathotypes with fewer deleterious mutations tend to reproduce more quickly. This results in a mutation–selection balance that determines the quasispecies distribution.
Figure 2
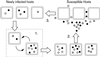
The horizontal transmission model. Starting with a population of n newly infected hosts, the transmission cycle proceeds as follows. (1) Within each host, the virus undergoes_t_ periods of intrahost process of replication and mutation. (2) Hosts carrying mature virus populations release viral particles into the environment. In this model, all hosts contribute viral particles, in proportion to their viral titer, to a common pool (e.g., a host carrying twice as many virions as a second host contributes twice as many viral particles to the common pool). (3) Susceptible hosts are infected by b viral particles drawn at random from this common pool, thereby generating a new set of newly infected hosts.
Figure 3
The vertical transmission model. (i) Within each of n newly infected hosts, the virus population undergoes t periods of mutation and growth, giving rise to a population of hosts carrying mature virus populations. (ii) A total of n newly infected offspring are produced by sampling with replacement from the set of “parent” hosts. Each offspring carries b virion particles drawn at random from the pathotype frequency distribution in its parent.
Figure 4
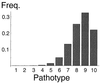
The pathotype distribution at the deterministic mutation-selection balance, given by the leading eigenvector of the matrix W.
Figure 5
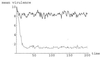
The change in mean pathotype over time in transmission events, for the horizontal (solid line) and the vertical (dashed line) transmission models with n = 25, t = 10, and b = 1. For comparison, the mean pathotype at the quasispecies equilibrium is 8.47.
Figure 6
The effect of host population size on the pathotype distributions. Long-term distributions of pathotype frequencies, generated by computer simulation, are shown for the vertical transmission model (shaded bars) and horizontal transmission model (open bars). Parameter values are t = 10 and_b_ = 1. (Upper Left) n = 1; (Upper Right) n = 5; (Lower Left)n = 10; (Lower Right) n = 25. The distributions shown are averaged over 1,800 transmission events, after a 200-generation settling period that allows the system to approach equilibrium.
Figure 7
The effect of the number of within-host viral replication events between each transmission event, on the pathotype distributions for n = 25 and b = 1 in the vertical (shaded bars) and horizontal (open bars) models. (Upper Left) t = 1; (Upper Right)t = 10; (Lower Left) t = 25; (Lower Right) t = 50. The pathotype distributions were generated by computer simulation; each histogram represents the pathotype distribution averaged over 45,000 viral replication periods, after an initial settling-time of 5,000 viral replication periods.
Figure 8
The effect of transmission bottleneck size on the pathotype distributions for n = 25 and t = 10 in the vertical (shaded bars) and horizontal (open bars) models. (Upper Left) b = 1; (Upper Right) b = 2; (Bottom Left)b = 4; (Bottom Right) b = 8. The simulation procedure is as described in Fig. 6.
Figure 9
The expected virulence after a single round of host-to-host transfer, starting at the deterministic mutation–selection balance distribution, for the vertical transmission model. Curves correspond to bottleneck sizes of 1, 2, 3, and 4. Horizontal line gives the mean virulence at the deterministic mutation-selection balance.
Similar articles
- Rapid fitness losses in mammalian RNA virus clones due to Muller's ratchet.
Duarte E, Clarke D, Moya A, Domingo E, Holland J. Duarte E, et al. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6015-9. doi: 10.1073/pnas.89.13.6015. Proc Natl Acad Sci U S A. 1992. PMID: 1321432 Free PMC article. - Evolution of sex and the molecular clock in RNA viruses.
Chao L. Chao L. Gene. 1997 Dec 31;205(1-2):301-8. doi: 10.1016/s0378-1119(97)00405-8. Gene. 1997. PMID: 9461404 Review. - Fitness of RNA virus decreased by Muller's ratchet.
Chao L. Chao L. Nature. 1990 Nov 29;348(6300):454-5. doi: 10.1038/348454a0. Nature. 1990. PMID: 2247152 - An empirical study of the evolution of virulence under both horizontal and vertical transmission.
Stewart AD, Logsdon JM Jr, Kelley SE. Stewart AD, et al. Evolution. 2005 Apr;59(4):730-9. Evolution. 2005. PMID: 15926685 - Impact of RNA Virus Evolution on Quasispecies Formation and Virulence.
Mandary MB, Masomian M, Poh CL. Mandary MB, et al. Int J Mol Sci. 2019 Sep 19;20(18):4657. doi: 10.3390/ijms20184657. Int J Mol Sci. 2019. PMID: 31546962 Free PMC article. Review.
Cited by
- Drift increases the advantage of sex in RNA bacteriophage Phi6.
Poon A, Chao L. Poon A, et al. Genetics. 2004 Jan;166(1):19-24. doi: 10.1534/genetics.166.1.19. Genetics. 2004. PMID: 15020402 Free PMC article. - Insights into arbovirus evolution and adaptation from experimental studies.
Ciota AT, Kramer LD. Ciota AT, et al. Viruses. 2010 Dec;2(12):2594-617. doi: 10.3390/v2122594. Epub 2010 Dec 2. Viruses. 2010. PMID: 21994633 Free PMC article. - Heterogeneous adaptive trajectories of small populations on complex fitness landscapes.
Rozen DE, Habets MG, Handel A, de Visser JA. Rozen DE, et al. PLoS One. 2008 Mar 5;3(3):e1715. doi: 10.1371/journal.pone.0001715. PLoS One. 2008. PMID: 18320036 Free PMC article. - Narrow bottlenecks affect Pea seedborne mosaic virus populations during vertical seed transmission but not during leaf colonization.
Fabre F, Moury B, Johansen EI, Simon V, Jacquemond M, Senoussi R. Fabre F, et al. PLoS Pathog. 2014 Jan;10(1):e1003833. doi: 10.1371/journal.ppat.1003833. Epub 2014 Jan 9. PLoS Pathog. 2014. PMID: 24415934 Free PMC article. - Ratcheting down the virulence of SARS-CoV-2 in the COVID-19 pandemic.
Brufsky A, Lotze MT. Brufsky A, et al. J Med Virol. 2020 Nov;92(11):2379-2380. doi: 10.1002/jmv.26067. Epub 2020 Jun 3. J Med Virol. 2020. PMID: 32458475 Free PMC article. No abstract available.
References
- Antia R, Levin B R, May R M. Am Nat. 1994;144:457–472.
- Bonhoeffer S, Nowak M A. Proc R Soc London Ser B. 1994;258:133–140.
- Bull J J, Molineaux I J, Rice W R. Evolution. 1991;45:875–882. - PubMed
- Ewald P W. Ann N Y Acad Sci. 1987;503:295–306. - PubMed
- Herre E A. Science. 1993;259:1442–1445. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources