Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes - PubMed (original) (raw)
Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes
K Liu et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Human telomerase consists of two essential components, telomerase RNA template (hTER) and telomerase reverse transcriptase (hTERT), and functions to synthesize telomere repeats that serve to protect the integrity of chromosomes and to prolong the replicative life span of cells. Telomerase activity is expressed selectively in germ-line and malignant tumor cells but not in most normal human somatic cells. As a notable exception, telomerase is expressed in human lymphocytes during development, differentiation, and activation. Recent studies have suggested that regulation of telomerase is determined by transcription of hTERT but not hTER. The highly regulated expression of telomerase in lymphocytes provides an opportunity to analyze the contribution of transcriptional regulation of hTERT and hTER. We report here an analysis of hTERT expression by Northern and in situ hybridization. It was found that hTERT mRNA is expressed at detectable levels in all subsets of human lymphocytes isolated from thymus, tonsil, and peripheral blood, regardless of the status of telomerase activity. hTERT expression is regulated as a function of lineage development, differentiation, and activation. Strikingly, however, telomerase activity in these cells is not correlated strictly with the levels of hTERT and hTER transcripts. The absence of correlation between telomerase activity and hTERT mRNA could not be attributed to the presence of hTERT splice variants or to detectable inhibitors of telomerase activity. Thus, transcriptional regulation of hTERT is not sufficient to account for telomerase activity in human lymphocytes, indicating a likely role of posttranscriptional factors in the control of enzyme function.
Figures
Figure 1
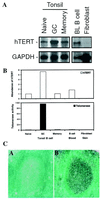
hTERT mRNA expression and telomerase activity in tonsil and peripheral-blood B cells. (A) Representative Northern blots of hTERT expression in naïve, GC, and memory B cells isolated from human tonsils, resting B cells isolated from peripheral blood, and cultured fibroblasts from neonatal foreskin are shown. The blots were probed sequentially with hTERT and GAPDH probes. (B) The relative abundance of hTERT mRNA and telomerase activity in B cell subsets. The hTERT level from each cell subset shown in A was analyzed by
phosphorimager
and
imagequant
software. The relative abundance of hTERT mRNA was normalized on the basis of cell equivalents. The open bars represent the relative abundance of hTERT expression for each cell subset. The level of hTERT in naïve cells was arbitrarily set at 1. Telomerase activity was measured by a modified TRAP assay from ≈104 cells, and the results were normalized on the basis of cell equivalents. The black bars represent the relative levels of telomerase activity. The level of telomerase in naïve cells was arbitrarily set at 1. (C) hTERT mRNA expression in tonsil section by i_n situ_ hybridization. The tonsil sections were probed with sense (C, A) and antisense probe (C, B). Strong expression of hTERT is observed in GC cells but not in surrounding naïve and memory B cells.
Figure 2
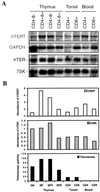
hTERT mRNA expression and telomerase activity in normal human T cells. (A) Representative Northern blot of hTERT and hTER expression in thymocytes, tonsil T cells, and peripheral-blood T cells is shown. The blots were probed sequentially with either hTERT and GAPDH or hTER and 7SK probes. (B) Comparison of relative abundance of hTERT and hTER expression and telomerase activity. The levels of hTERT and hTER from each cell subset were determined by
imagequant
software. The relative abundance of hTERT mRNA and hTER were calculated by normalizing on the basis of cell equivalents. The bars represent relative abundance of hTERT (open bars) and hTER (gray bars) transcripts for each cell subset. The level of hTERT and hTER in double-negative (DN) thymocyte was arbitrarily set at 1. Telomerase activity was measured by a modified TRAP assay as described (33). The black bars represent the relative telomerase activity. The level of telomerase enzymatic activity in the CD4−CD8− subset was arbitrarily set at 1. DP, double-positive; SP, single-positive.
Figure 3
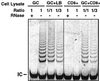
Absence of detectable telomerase inhibitory effect in mixtures of cell lysates from telomerase-negative and telomerase-positive cells. Cell lysate from telomerase-positive GC B cells was mixed with either cell lysis buffer or cell lysates from telomerase-negative blood CD8+ T cells at ratios of 1:1 or 1:3, respectively. The specificity of the assay was controlled by RNase treatment of cell lysates and by inclusion of a 36-bp internal control for PCR amplification. GC, GC B cells; B, peripheral-blood resting CD8+ T cells; LB, cell lysis buffer; IC, internal control.
Figure 4
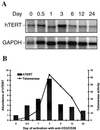
hTERT mRNA expression in peripheral-blood CD4+ T cells during in vitro activation. (A) Representative Northern blot of hTERT mRNA expression in peripheral-blood CD4+ T cells after in vitro stimulation with anti-CD3 and anti-CD28 antibodies. Peripheral-blood CD4+ T cells were isolated from healthy donors, stimulated with anti-CD3 plus anti-CD28, and cultured for various time intervals up to 24 days. The Northern blots were probed sequentially with hTERT and GAPDH probes. (B) Comparison of relative abundance of hTERT mRNA and telomerase activity. The hTERT mRNA levels at each time point were determined by
phosphorimager
and
imagequant
software. The relative abundance of hTERT mRNA was normalized to the number of cell equivalents used to isolate mRNA. The bars represent relative abundance of hTERT expression at each given time point; the level before the stimulation (time 0) was set at 1. Telomerase activity was measured by a modified TRAP assay as described (33). The line represents the relative telomerase activity; the level before stimulation was set at 1.
Figure 5
RT-PCR analysis of hTERT expression in B and T lymphocytes. (Upper) Representative result of the RT-PCR analysis of B and T lymphocyte hTERT expression with primers hTERT-2164S and hTERT-2620A (35). The amplified products were separated on a 2.0% agarose gel and visualized by ethidium bromide staining. The α- and β-deletions of hTERT result in a loss of 36 bp and 182 bp, respectively. (Lower) Representative result of RT-PCR analysis of B and T lymphocyte hTERT expression with primers hTERT-1680S and hTERT-2974A, which cover the entire region of reverse transcriptase. The amplified products were separated on a 1.2% agarose gel and visualized by ethidium bromide staining. The DNA molecular weight markers were run alongside the samples and indicated at the left.
Similar articles
- Human normal T lymphocytes and lymphoid cell lines do express alternative splicing variants of human telomerase reverse transcriptase (hTERT) mRNA.
Jalink M, Ge Z, Liu C, Björkholm M, Gruber A, Xu D. Jalink M, et al. Biochem Biophys Res Commun. 2007 Feb 23;353(4):999-1003. doi: 10.1016/j.bbrc.2006.12.149. Epub 2006 Dec 27. Biochem Biophys Res Commun. 2007. PMID: 17204238 - Human telomerase reverse transcriptase promoter regulation in normal and malignant human ovarian epithelial cells.
Braunstein I, Cohen-Barak O, Shachaf C, Ravel Y, Yalon-Hacohen M, Mills GB, Tzukerman M, Skorecki KL. Braunstein I, et al. Cancer Res. 2001 Jul 15;61(14):5529-36. Cancer Res. 2001. PMID: 11454703 - Regulation of the human telomerase reverse transcriptase gene.
Ducrest AL, Szutorisz H, Lingner J, Nabholz M. Ducrest AL, et al. Oncogene. 2002 Jan 21;21(4):541-52. doi: 10.1038/sj.onc.1205081. Oncogene. 2002. PMID: 11850779 Review. - Physiological and pathological significance of human telomerase reverse transcriptase splice variants.
Bollmann FM. Bollmann FM. Biochimie. 2013 Nov;95(11):1965-70. doi: 10.1016/j.biochi.2013.07.031. Epub 2013 Aug 9. Biochimie. 2013. PMID: 23933091 Review.
Cited by
- Anti-cancer Immunotherapies Targeting Telomerase.
Negrini S, De Palma R, Filaci G. Negrini S, et al. Cancers (Basel). 2020 Aug 12;12(8):2260. doi: 10.3390/cancers12082260. Cancers (Basel). 2020. PMID: 32806719 Free PMC article. Review. - Expression profile of telomere-associated genes in multiple myeloma.
Díaz de la Guardia R, Catalina P, Panero J, Elosua C, Pulgarin A, López MB, Ayllón V, Ligero G, Slavutsky I, Leone PE. Díaz de la Guardia R, et al. J Cell Mol Med. 2012 Dec;16(12):3009-21. doi: 10.1111/j.1582-4934.2012.01628.x. J Cell Mol Med. 2012. PMID: 22947336 Free PMC article. - Telomerase immunity from bench to bedside: round one.
Cortez-Gonzalez X, Zanetti M. Cortez-Gonzalez X, et al. J Transl Med. 2007 Feb 26;5:12. doi: 10.1186/1479-5876-5-12. J Transl Med. 2007. PMID: 17324292 Free PMC article. Review. - Telomeres and immunological diseases of aging.
Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Andrews NP, et al. Gerontology. 2010;56(4):390-403. doi: 10.1159/000268620. Epub 2009 Dec 17. Gerontology. 2010. PMID: 20016137 Free PMC article. Review. - Transcriptional activation of hTERT, the human telomerase reverse transcriptase, by nuclear factor of activated T cells.
Chebel A, Rouault JP, Urbanowicz I, Baseggio L, Chien WW, Salles G, Ffrench M. Chebel A, et al. J Biol Chem. 2009 Dec 18;284(51):35725-34. doi: 10.1074/jbc.M109.009183. J Biol Chem. 2009. PMID: 19843528 Free PMC article.
References
- Blackburn E H. Annu Rev Biochem. 1992;61:113–129. - PubMed
- Nakamura T M, Cech T R. Cell. 1998;92:587–590. - PubMed
- de Lange T. Science. 1998;279:334–335. - PubMed
- Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials