The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1 - PubMed (original) (raw)
The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1
J C Reeder et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Chondroitin sulfate A (CSA) is an important receptor for the sequestration of Plasmodium falciparum in the placenta, but the parasite ligand involved in adhesion has not previously been identified. Here we report the identification of a var gene transcribed in association with binding to CSA and present evidence that the P. falciparum erythrocyte membrane protein 1 product of the gene is the parasite ligand mediating CSA binding. Description of this gene and the implication of P. falciparum erythrocyte membrane protein 1 as the parasite ligand paves the way to a more detailed understanding of the pathogenesis of placental infection and potential therapeutic strategies targeting the interaction.
Figures
Figure 1
A differentially expressed var gene associated with CSA binding was detected and sequenced. (a) Degenerate universal primers amplified a var gene DBL3 region from genomic DNA of parent and selected line, and from cDNA only in the CSA binding line. The related EBA-175 gene also was amplified under these conditions. (b) The differential expression of the cDNA transcript was confirmed with sequence specific primers. (c) Full-length sequence was obtained for a _var_-CS2 gene, including three DBL domains. The approximate positions of the primers used to generate a gene-specific probe are marked with arrows. SVL, sequence of variable length. The lines below the gene represent nine different regions that were expressed as fusion proteins and to which rabbit antisera were raised.
Figure 2
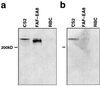
Antibody to recombinant fusion proteins demonstrates that a different form of PfEMP1 is expressed in the CSA selected line. (a) Western blot from a reducing PAGE probed with the conserved anti-ATS affinity purified serum showing size differences in PfEMP1. (b) Western blot from a nonreducing PAGE probed with anti-DBL4 serum differentiates between CS2 and FAF-EA8 PfEMP1.
Figure 3
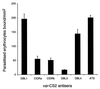
Antisera to the recombinant _var_-CS2 CIDR and DBL3 domains significantly inhibit the binding of CS2 to CSA, when compared with DBL1, DBL4, or ATS. The bars represent the mean (±SEM) numbers of CS2-parasitized erythrocytes binding per mm2 to immobilized CSA, determined by triplicate assays performed on four separate occasions.
Similar articles
- Cross-reactive surface epitopes on chondroitin sulfate A-adherent Plasmodium falciparum-infected erythrocytes are associated with transcription of var2csa.
Elliott SR, Duffy MF, Byrne TJ, Beeson JG, Mann EJ, Wilson DW, Rogerson SJ, Brown GV. Elliott SR, et al. Infect Immun. 2005 May;73(5):2848-56. doi: 10.1128/IAI.73.5.2848-2856.2005. Infect Immun. 2005. PMID: 15845490 Free PMC article. - Placenta cryosections for study of the adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A in flow conditions.
Avril M, Traoré B, Costa FT, Lépolard C, Gysin J. Avril M, et al. Microbes Infect. 2004 Mar;6(3):249-55. doi: 10.1016/j.micinf.2003.12.007. Microbes Infect. 2004. PMID: 15026011 - [Plasmodium falciparum and chondroitin-4-sulfate: the new key couple in sequestration].
Pouvelle B, Fusai T, Gysin J. Pouvelle B, et al. Med Trop (Mars). 1998;58(2):187-98. Med Trop (Mars). 1998. PMID: 9791601 Review. French. - Molecular mechanisms of Plasmodium falciparum placental adhesion.
Scherf A, Pouvelle B, Buffet PA, Gysin J. Scherf A, et al. Cell Microbiol. 2001 Mar;3(3):125-31. doi: 10.1046/j.1462-5822.2001.00109.x. Cell Microbiol. 2001. PMID: 11260135 Review.
Cited by
- Mechanical forces control the valency of the malaria adhesin VAR2CSA by exposing cryptic glycan binding sites.
Roessner R, Michelarakis N, Gräter F, Aponte-Santamaría C. Roessner R, et al. PLoS Comput Biol. 2023 Dec 20;19(12):e1011726. doi: 10.1371/journal.pcbi.1011726. eCollection 2023 Dec. PLoS Comput Biol. 2023. PMID: 38117828 Free PMC article. - Antigenic differences and conservation among placental Plasmodium falciparum-infected erythrocytes and acquisition of variant-specific and cross-reactive antibodies.
Beeson JG, Mann EJ, Byrne TJ, Caragounis A, Elliott SR, Brown GV, Rogerson SJ. Beeson JG, et al. J Infect Dis. 2006 Mar 1;193(5):721-30. doi: 10.1086/500145. Epub 2006 Jan 30. J Infect Dis. 2006. PMID: 16453269 Free PMC article. - A novel murine model for assessing fetal and birth outcomes following transgestational maternal malaria infection.
Morffy Smith CD, Russ BN, Andrew AK, Cooper CA, Moore JM. Morffy Smith CD, et al. Sci Rep. 2019 Dec 20;9(1):19566. doi: 10.1038/s41598-019-55588-8. Sci Rep. 2019. PMID: 31862902 Free PMC article. - Six genes are preferentially transcribed by the circulating and sequestered forms of Plasmodium falciparum parasites that infect pregnant women.
Francis SE, Malkov VA, Oleinikov AV, Rossnagle E, Wendler JP, Mutabingwa TK, Fried M, Duffy PE. Francis SE, et al. Infect Immun. 2007 Oct;75(10):4838-50. doi: 10.1128/IAI.00635-07. Epub 2007 Aug 13. Infect Immun. 2007. PMID: 17698567 Free PMC article.
References
- Aikawa M, Iseki M, Barnwell J W, Taylor D, Oo M M, Howard R J. Am J Trop Med Hyg. 1990;43:30–37. - PubMed
- Bulmer J N, Rasheed F N, Francis N, Morrison L, Greenwood B M. Histopathology. 1993;22:211–218. - PubMed
- Ockenhouse C F, Tandon N N, Magowan C, Jamieson G A, Chulay J D. Science. 1989;243:1469–1471. - PubMed
- Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Nature (London) 1989;341:57–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials