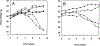Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo - PubMed (original) (raw)
Comparative Study
Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo
M H Hanigan et al. Carcinogenesis. 1999 Apr.
Abstract
We have shown previously that gamma-glutamyl transpeptidase (GGT) activity is essential for the nephrotoxicity of cisplatin. In this study we asked whether GGT activity was necessary for the antitumor activity of cisplatin. GGT was transfected into PC3 cells, a human prostate tumor cell line. Two independent GGT-positive cell lines were isolated and characterized. GGT cleaves extracellular glutathione providing the cells with access to additional cysteine. Expression of GGT had no effect on the growth rate of the cells in vitro where the culture medium contains high levels of cysteine. However, when the cells were injected into nude mice the GGT-positive tumors grew at more than twice the rate of the GGT-negative tumors. Weekly treatment with cisplatin was toxic to both GGT-positive and -negative tumors. The GGT-positive tumors were significantly more resistant to the toxicity of cisplatin than the GGT-negative tumors. Therefore, expression of GGT is required for the nephrotoxicity of cisplatin, but diminishes the tumor toxicity of the drug. These results indicate that the nephrotoxicity and the tumor toxicity of cisplatin are via two distinct pathways.
Figures
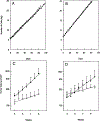
Fig. 1.
Growth of GGT-positive and -negative PC3 cells in vitro and in vivo. Growth in vitro of GGT-positive, PC3 cells (+), GGT-negative, PC3 cells (○) and parental PC3 cells (△) in experiment 1 (A) and experiment 2 (B). Growth of the same PC3 GGT-positive cells (●) and GGT-negative cells (s) when injected into nude mice, experiment 1 (C); experiment 2 (D).
Fig. 2.
Weight of mice during treatment with cisplatin. Mice bearing GGT-positive, PC3 tumors (closed symbols) and GGT-negative PC3 tumors (open symbols) were weighed weekly prior to injection with saline (circles), 2.5 mg/kg cisplatin (squares) or 5.0 mg/kg cisplatin (triangles) in experiment 1 (A) and experiment 2 (B).
Fig. 3.
Immunohistochemical staining of tumors derived from PC3/GGT1 cells (A) and PC3/GGT2 cells (B). Tumors were stained with GGT129, an antibody directed against a 20 amino acid sequence in the human GGT protein. Antibody staining in the controls, PC3/C1 and PC3/C2 derived tumors, was negative (data not shown).
Similar articles
- Controversial role of gamma-glutamyl transferase activity in cisplatin nephrotoxicity.
Fliedl L, Wieser M, Manhart G, Gerstl MP, Khan A, Grillari J, Grillari-Voglauer R. Fliedl L, et al. ALTEX. 2014;31(3):269-78. doi: 10.14573/altex.1311152. Epub 2014 Mar 21. ALTEX. 2014. PMID: 24664430 - gamma-Glutamyl transpeptidase catalyses the extracellular detoxification of cisplatin in a human cell line derived from the proximal convoluted tubule of the kidney.
Paolicchi A, Sotiropuolou M, Perego P, Daubeuf S, Visvikis A, Lorenzini E, Franzini M, Romiti N, Chieli E, Leone R, Apostoli P, Colangelo D, Zunino F, Pompella A. Paolicchi A, et al. Eur J Cancer. 2003 May;39(7):996-1003. doi: 10.1016/s0959-8049(03)00067-4. Eur J Cancer. 2003. PMID: 12706370 - Gamma-glutamyl transpeptidase-deficient mice are resistant to the nephrotoxic effects of cisplatin.
Hanigan MH, Lykissa ED, Townsend DM, Ou CN, Barrios R, Lieberman MW. Hanigan MH, et al. Am J Pathol. 2001 Nov;159(5):1889-94. doi: 10.1016/s0002-9440(10)63035-0. Am J Pathol. 2001. PMID: 11696449 Free PMC article. - Gamma-glutamyl transpeptidase: redox regulation and drug resistance.
Hanigan MH. Hanigan MH. Adv Cancer Res. 2014;122:103-41. doi: 10.1016/B978-0-12-420117-0.00003-7. Adv Cancer Res. 2014. PMID: 24974180 Free PMC article. Review. - gamma-Glutamyl transpeptidase, a glutathionase: its expression and function in carcinogenesis.
Hanigan MH. Hanigan MH. Chem Biol Interact. 1998 Apr 24;111-112:333-42. doi: 10.1016/s0009-2797(97)00170-1. Chem Biol Interact. 1998. PMID: 9679564 Review.
Cited by
- Association of gamma-glutamyltransferase and risk of cancer incidence in men: a prospective study.
Strasak AM, Rapp K, Brant LJ, Hilbe W, Gregory M, Oberaigner W, Ruttmann E, Concin H, Diem G, Pfeiffer KP, Ulmer H; VHM&PP Study Group. Strasak AM, et al. Cancer Res. 2008 May 15;68(10):3970-7. doi: 10.1158/0008-5472.CAN-07-6686. Cancer Res. 2008. PMID: 18483283 Free PMC article. - Inhibition of human γ-glutamyl transpeptidase: development of more potent, physiologically relevant, uncompetitive inhibitors.
Wickham S, Regan N, West MB, Thai J, Cook PF, Terzyan SS, Li PK, Hanigan MH. Wickham S, et al. Biochem J. 2013 Mar 15;450(3):547-57. doi: 10.1042/BJ20121435. Biochem J. 2013. PMID: 23301618 Free PMC article. - High pretreatment serum gamma-glutamyl transpeptidase predicts an inferior outcome in nasopharyngeal carcinoma.
Luo M, Sun W, Wu C, Zhang L, Liu D, Li W, Mei Q, Hu G. Luo M, et al. Oncotarget. 2017 Jun 28;8(40):67651-67662. doi: 10.18632/oncotarget.18798. eCollection 2017 Sep 15. Oncotarget. 2017. PMID: 28978060 Free PMC article. - Sulfur-containing histidine compounds inhibit γ-glutamyl transpeptidase activity in human cancer cells.
Brancaccio M, Russo M, Masullo M, Palumbo A, Russo GL, Castellano I. Brancaccio M, et al. J Biol Chem. 2019 Oct 4;294(40):14603-14614. doi: 10.1074/jbc.RA119.009304. Epub 2019 Aug 2. J Biol Chem. 2019. PMID: 31375562 Free PMC article.
References
- Colvin OM (1997) Alkylating agents and platinum antitumor compounds. In Holland JF, Bast RC Jr, Morton DL, Frei E III, Kufe DW and Weichselbaum RR (eds) Cancer Medicine Williams and Williams, Baltimore, MD, pp. 949–975.
- Hanigan MH (1998) Gamma-glutamyl transpeptidase, a glutathionease: its expression and function in carcinogenesis. Chem. Biol. Interact, 111–112, 333–342. - PubMed
- Hanigan MH and Ricketts WA (1993) Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry, 32, 6302–6306. - PubMed
- Elfarra AA and Anders MW (1984) Renal processing of glutathione conjugates, role in nephrotoxicity. Biochem. Pharmacol, 33, 3729–3732. - PubMed
- Hanigan MH, Gallagher BC, Taylor PT Jr and Large MK (1994) Inhibition of gamma-glutamyl transpeptidase activity by acivicin in vivo protects the kidney from cisplatin-induced toxicity. Cancer Res, 54, 5925–5929. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous