Shiga toxin 1 from Escherichia coli blocks activation and proliferation of bovine lymphocyte subpopulations in vitro - PubMed (original) (raw)
Shiga toxin 1 from Escherichia coli blocks activation and proliferation of bovine lymphocyte subpopulations in vitro
C Menge et al. Infect Immun. 1999 May.
Abstract
Shiga toxin-producing Escherichia coli (STEC) is widespread in the cattle population, but the clinical significance of Shiga toxins (Stx's) for the bovine species remains obscure. Since Stx's exert immunomodulating effects in other species, we examined the effect of purified Stx1 on a bovine B lymphoma cell line (BL-3) and peripheral blood mononuclear cells (PBMC) isolated from adult bovine blood by viability assays and flow cytometry analysis. Stx1 markedly induced apoptosis in stimulated BL-3 cells. The susceptibility of this B-cell-derived cell line was induced only by either lipopolysaccharide (LPS) or pokeweed mitogen, while cultures stimulated with T-cell mitogens were unaffected by the toxin. In contrast, Stx1 did not induce cellular death-neither apoptosis nor necrosis-in primary cultures of PBMC but hindered the mitogen-induced increase in metabolic activity. The influence of Stx1 on single PBMC subpopulations varied with the type of mitogenic stimulus applied. Stimulation with phytohemagglutinin P particularly induced the proliferation of bovine CD8-expressing (BoCD8(+)) cells, and this proliferative response was blocked by Stx1. On the other hand, Stx1 reduced the portion of viable B cells in the presence of LPS. Modulation of activation marker expression (BoCD25 and BoCD71) by Stx1 indicated that the toxin hindered the proliferation of cells by blocking their activation. In conclusion, we assume that Stx1 contributes to the pathogenesis of STEC-associated diarrhea in calves by suppressing the mucosa-associated immune response. The usefulness of cattle as a model in which to study Stx-induced immunomodulation is discussed.
Figures
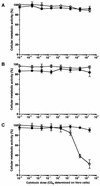
FIG. 1
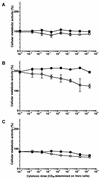
Effect of purified Stx1 on the cellular metabolic activity of BL-3 cells. Cells were incubated with 10-fold dilutions of purified Stx1 (0.002 to 2,000 CD50/ml; quantified on Vero cells as described in Materials and Methods) for 96 h at 37°C. The culture medium was free of mitogen (A) or was supplemented with 5 μg of PHA-P/ml (B) or with 25 μg of LPS/ml (C). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence (open circles) or presence (filled circles) of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. Cellular metabolic activity was determined by MTT reduction assay. Cells incubated with medium alone were used as a negative control, while cells treated with 1% SDS served as a positive control to calculate percent activity. Data are means ± standard deviations from triplicate determinations. Missing error bars are within symbols. Two-way ANOVA revealed significances only for the curves presented in graph C (P ≤ 0.001 for anti-StxB1; P ≤ 0.001 for the concentration of Stx1).
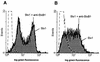
FIG. 2
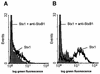
Representative flow cytometric histograms illustrating the induction of DNA strand breaks in BL-3 cells by Stx1. Cells were treated with 200 CD50/ml (quantified on Vero cells as described in Materials and Methods) for 96 h at 37°C. The culture medium was free of mitogens (A) or was supplemented with 25 μg of LPS/ml (B). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence or presence of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. After incubation, DNA strand breaks were labeled by the TUNEL method. Stx1 was able to induce DNA strand breaks only in LPS-treated BL-3 cells. Histograms are from one representative of two experiments.
FIG. 3
Effect of purified Stx1 on the cellular metabolic activity of bovine PBMC. Cells were incubated with 10-fold dilutions of purified Stx1 (0.002 to 2,000 CD50/ml; quantified on Vero cells as described in Materials and Methods) for 96 h at 37°C. The culture medium was free of mitogens (A) or was supplemented with 5 μg of PHA-P/ml (B) or 25 μg of LPS/ml (C). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence (open circles) or presence (filled circles) of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. Cellular metabolic activity was determined by MTT reduction assay. Cells incubated with medium alone were used as a negative control, while cells treated with 1% SDS served as a positive control to calculate percent activity. Data are means ± standard deviations of triplicate determinations from one representative of six independent experiments. Missing error bars are within symbols. Two-way ANOVA revealed significances for all curves in all panels (P ≤ 0.001 for anti-StxB1 and P ≤ 0.001 for the concentration of Stx1).
FIG. 4
Representative flow cytometric histograms illustrating DNA strand breaks in bovine PBMC. Cells were treated with 200 CD50/ml (quantified on Vero cells as described in Materials and Methods) for 96 h at 37°C. The culture medium was free of mitogens (A) or was supplemented with 5 μg of PHA-P/ml (B). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence or presence of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. After incubation DNA strand breaks were labeled by the TUNEL method. Stx1 was not able to induce DNA strand breaks either in unstimulated or in PHA-P-stimulated PBMC. Histograms are from one representative of six experiments.
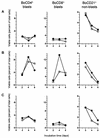
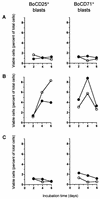
FIG. 5
Effect of purified Stx1 on transformation and proliferation of PBMC subpopulations. Cells were incubated with purified Stx1 (200 CD50/ml; quantified on Vero cells as described in Materials and Methods) at 37°C. The culture medium was free of mitogens (A) or was supplemented with 5 μg of PHA-P/ml (B) or 25 μg of LPS/ml (C). Observed effects were assigned to Stx1 by comparison of the results obtained in the absence (open circles) or presence (filled circles) of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. Lymphocyte subpopulations were identified by immunophenotyping at the time points indicated and quantified by flow cytometry acquiring 5,000 events. Data analysis was performed by using the software of the instrument to calculate the percentages of viable (PI-negative), immunolabeled events belonging to the blast cell or non-blast cell population. Data are single determinations from one representative of five independent experiments.
FIG. 6
Effect of purified Stx1 on expression of activation markers by bovine PBMC in vitro. Cells were incubated with purified Stx1 (200 CD50/ml; quantified on Vero cells as described in Materials and Methods) at 37°C. The culture medium was free of mitogens (A) or was supplemented with 5 μg of PHA-P/ml (B) or 25 μg of LPS/ml (C). Observed effects were assigned to Stx1 by comparison of results obtained in the absence (open circles) or presence (filled circles) of 1.5 μg of the monoclonal anti-StxB1 antibody 13C4/ml. Lymphocyte subpopulations were identified by immunophenotyping at the time points indicated and quantified by flow cytometry acquiring 5,000 events. Data analysis was performed by using the software of the instrument to calculate the percentage of viable (PI-negative), immunolabeled events belonging to the blast cell population.
Similar articles
- Antiviral activity of shiga toxin 1: suppression of bovine leukemia virus-related spontaneous lymphocyte proliferation.
Ferens WA, Hovde CJ. Ferens WA, et al. Infect Immun. 2000 Aug;68(8):4462-9. doi: 10.1128/IAI.68.8.4462-4469.2000. Infect Immun. 2000. PMID: 10899843 Free PMC article. - Comparison of binding and effects of Escherichia coli Shiga toxin 1 on bovine and ovine granulocytes.
Menge C, Eisenberg T, Stamm I, Baljer G. Menge C, et al. Vet Immunol Immunopathol. 2006 Oct 15;113(3-4):392-403. doi: 10.1016/j.vetimm.2006.06.009. Epub 2006 Aug 1. Vet Immunol Immunopathol. 2006. PMID: 16884782 - Bovine macrophages sense Escherichia coli Shiga toxin 1.
Menge C, Loos D, Bridger PS, Barth S, Werling D, Baljer G. Menge C, et al. Innate Immun. 2015 Aug;21(6):655-64. doi: 10.1177/1753425915581215. Epub 2015 Apr 23. Innate Immun. 2015. PMID: 25907071 - Shiga/verocytotoxins and Shiga/verotoxigenic Escherichia coli in animals.
Mainil J. Mainil J. Vet Res. 1999 Mar-Jun;30(2-3):235-57. Vet Res. 1999. PMID: 10367357 Review. - Shiga toxin-producing Escherichia coli: an overview.
Gyles CL. Gyles CL. J Anim Sci. 2007 Mar;85(13 Suppl):E45-62. doi: 10.2527/jas.2006-508. Epub 2006 Nov 3. J Anim Sci. 2007. PMID: 17085726 Review.
Cited by
- Bovine immune response to shiga-toxigenic Escherichia coli O157:H7.
Hoffman MA, Menge C, Casey TA, Laegreid W, Bosworth BT, Dean-Nystrom EA. Hoffman MA, et al. Clin Vaccine Immunol. 2006 Dec;13(12):1322-7. doi: 10.1128/CVI.00205-06. Epub 2006 Oct 18. Clin Vaccine Immunol. 2006. PMID: 17050743 Free PMC article. - Bovine ileal intraepithelial lymphocytes represent target cells for Shiga toxin 1 from Escherichia coli.
Menge C, Blessenohl M, Eisenberg T, Stamm I, Baljer G. Menge C, et al. Infect Immun. 2004 Apr;72(4):1896-905. doi: 10.1128/IAI.72.4.1896-1905.2004. Infect Immun. 2004. PMID: 15039308 Free PMC article. - Intimin, tir, and shiga toxin 1 do not influence enteropathogenic responses to shiga toxin-producing Escherichia coli in bovine ligated intestinal loops.
Stevens MP, Marchès O, Campbell J, Huter V, Frankel G, Phillips AD, Oswald E, Wallis TS. Stevens MP, et al. Infect Immun. 2002 Feb;70(2):945-52. doi: 10.1128/IAI.70.2.945-952.2002. Infect Immun. 2002. PMID: 11796630 Free PMC article. - Optimizing the Protection of Cattle against Escherichia coli O157:H7 Colonization through Immunization with Different Combinations of H7 Flagellin, Tir, Intimin-531 or EspA.
McNeilly TN, Mitchell MC, Corbishley A, Nath M, Simmonds H, McAteer SP, Mahajan A, Low JC, Smith DG, Huntley JF, Gally DL. McNeilly TN, et al. PLoS One. 2015 May 28;10(5):e0128391. doi: 10.1371/journal.pone.0128391. eCollection 2015. PLoS One. 2015. PMID: 26020530 Free PMC article. - Lysogeny with Shiga toxin 2-encoding bacteriophages represses type III secretion in enterohemorrhagic Escherichia coli.
Xu X, McAteer SP, Tree JJ, Shaw DJ, Wolfson EB, Beatson SA, Roe AJ, Allison LJ, Chase-Topping ME, Mahajan A, Tozzoli R, Woolhouse ME, Morabito S, Gally DL. Xu X, et al. PLoS Pathog. 2012;8(5):e1002672. doi: 10.1371/journal.ppat.1002672. Epub 2012 May 17. PLoS Pathog. 2012. PMID: 22615557 Free PMC article.
References
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol Suppl. 1976;5:9–15. - PubMed
- Cohen A, Madrid-Marina V, Estrov Z, Freedman M H, Lingwood C A, Dosch H M. Expression of glycolipid receptors to Shiga-like toxin on human B lymphocytes: a mechanism for the failure of long-lived antibody response to dysenteric disease. Int Immunol. 1990;2:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials